2025 AIChE Annual Meeting
(73b) In Vitro Evaluation of Osteoarthritic Inflammatory Models on Mesenchymal Stromal Cell Differentiation
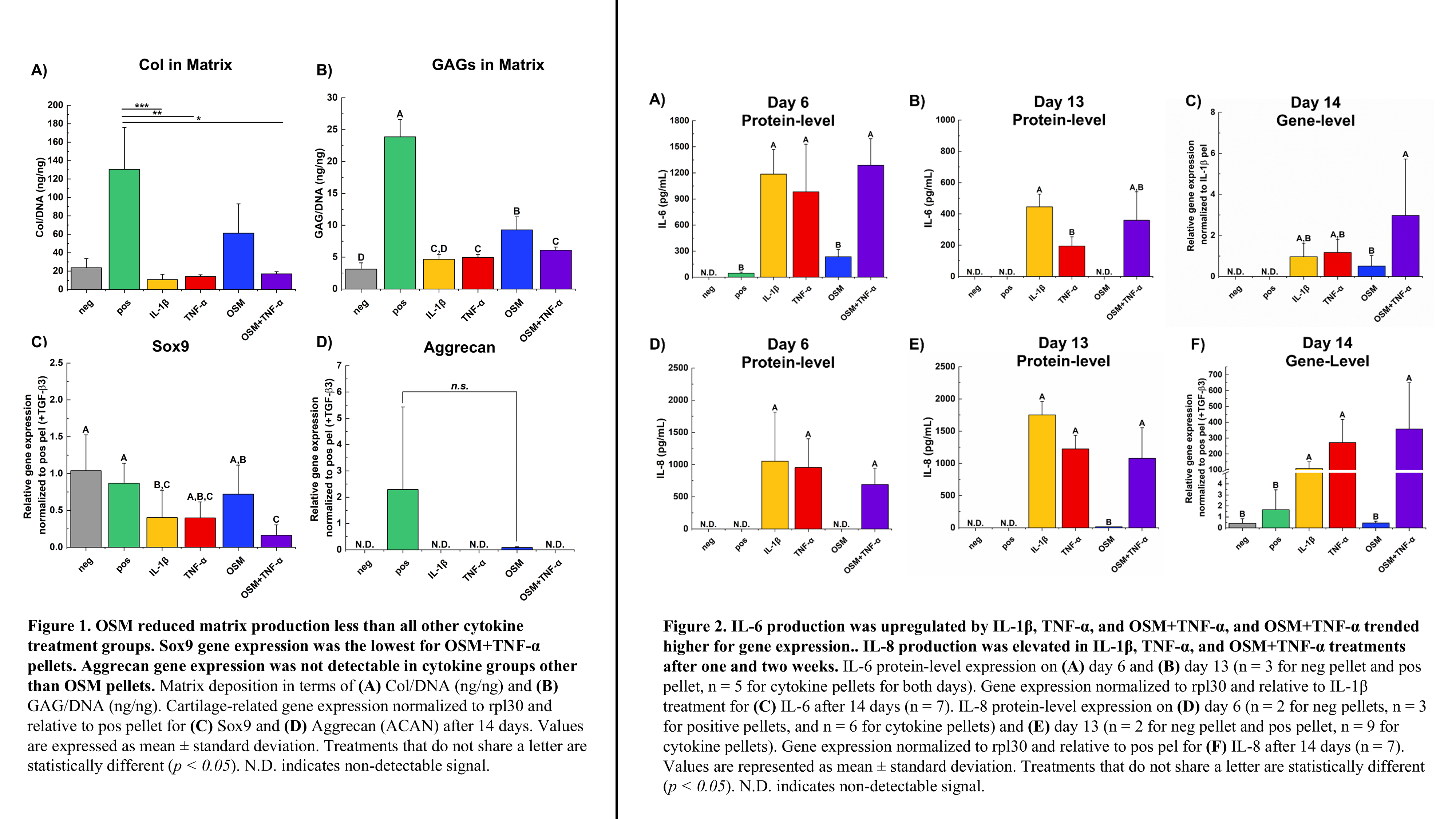
IL-1β, TNF-α, and OSM+TNF-α all produced a limited cartilage matrix (i.e., GAG and collagen production), whereas OSM alone did not limit production to the same extent. This observation was consistent with immunostaining of aggrecan and collagen: qualitatively less signal was present in IL-1β, TNF-α, and OSM+TNF-α groups. Similarly, these three treatment groups trended lower for Sox9 gene expression compared to positive controls. However, only OSM+TNF-α was statistically lower than positive. Additionally, only positive control pellets and OSM resulted in detectable aggrecan gene expression. IL-1β and OSM+TNF-α led to sustained levels of pro-inflammatory cytokine production, IL-6 and IL-8. However, OSM+TNF-α resulted in a more reproducible effect with less variation across samples. All cytokine treatments exhibited IL-6 gene expression, and OSM+TNF-α expression of IL-6 trended higher than other treatments. TNF-α increased both IL-6 and IL-8 production after one week, but the effect was not sustained as signal was not detectable after two weeks in culture. OSM did not result in detectable signal of IL-6 or IL-8 but did significantly increase MMP-13 gene and protein expression to a much greater extent than all other cytokine treatment groups. Negative and positive controls had no signal for any inflammatory markers in the media or gene expression. Smad7 immunostaining was more apparent in OSM treated samples.
Taken together, this work highlights that current in vitro models only capture certain OA disease outputs (e.g., IL-6, MMP-13 production) and that outputs vary depending on the environment. Here, the combination of OSM+TNF-α for assessment of treatments with MSCs in vitro captured various aspects of the osteoarthritic diseased state. OSM+TNF-α captured the significant downregulation of pro-cartilage formation genes (SOX9 and aggrecan) while also showing the elevated inflammatory response. Future tissue engineered constructs should be evaluated in the OSM+TNF-α mimic of the osteoarthritic environment to allow for more clinically relevant testing of these constructs.