2025 AIChE Annual Meeting
(327a) Upcycling of Polyester Wastes into Biodegradable Thermoplastics: Process Development and Analyses
Authors
Various solutions have been proposed to address the issue of waste plastic treatment. Among these, recycling is often considered the most intuitive approach. Recycling methods can be broadly categorized into thermal, mechanical (physical), and chemical recycling. Thermal recycling involves the combustion of waste plastics to generate heat and electricity. However, whether this method should be classified as a form of recycling remains a topic of ongoing debate, with prevailing views suggesting that it should not be regarded as true recycling. Mechanical recycling involves reshaping waste plastics into new materials through physical processing. In contrast, chemical recycling refers to the breakdown of plastics at the molecular level through chemical reactions, enabling the recovery and reuse of the constituent materials.
Most ongoing research on plastic recycling focuses on chemical recycling, wherein polymeric plastic structures are decomposed into monomers, which are then repolymerized to form new polymers. However, this polymer-to-monomer-to-polymer approach involves significant consumption of energy and chemicals, leading to economic drawbacks.
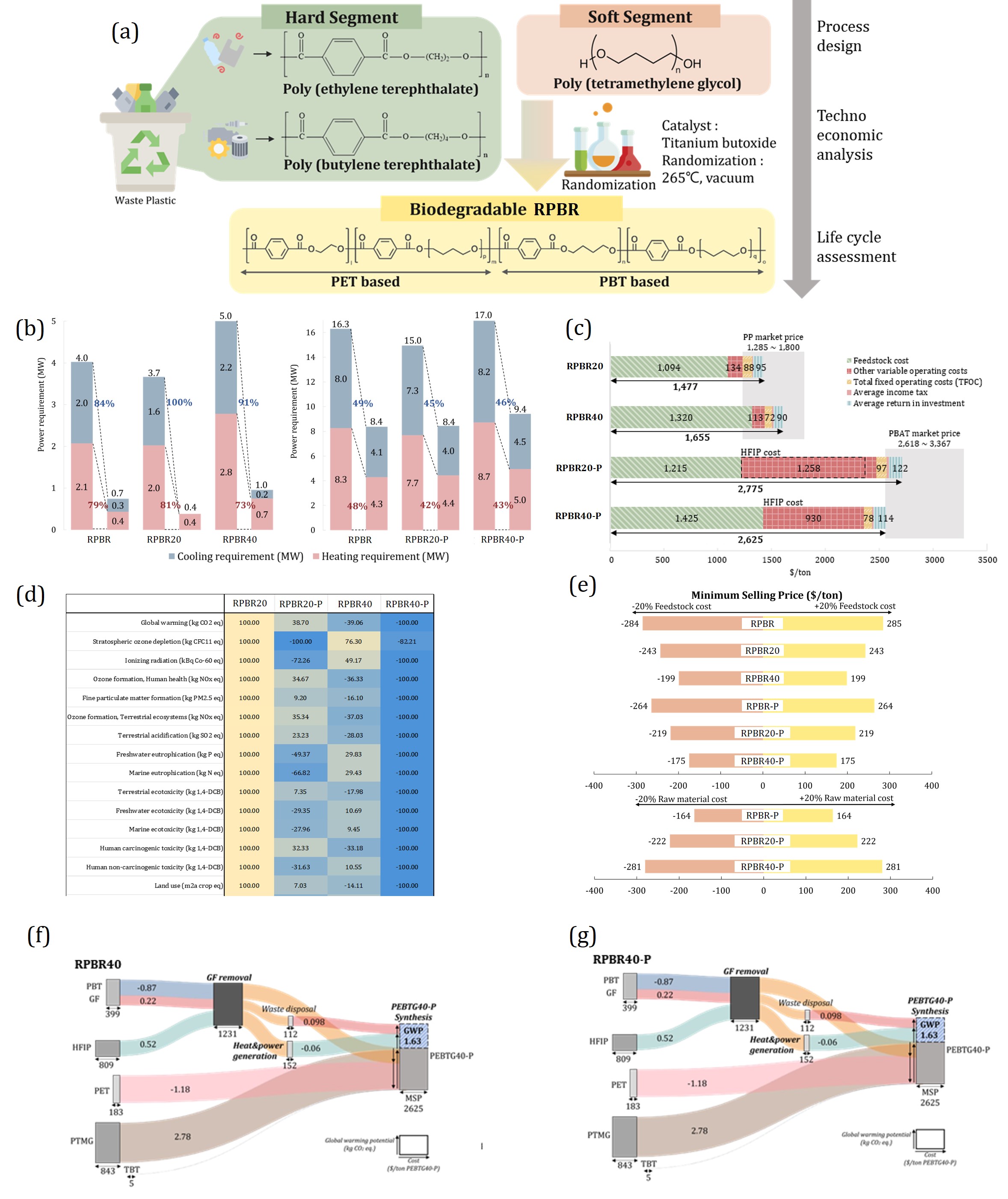
In this study, an alternative method is proposed that synthesizes new polymers directly from waste plastics in their polymeric form. This approach aims to reduce both energy and chemical usage compared to conventional chemical recycling, thereby minimizing associated economic and environmental impacts. To evaluate the proposed process, a techno-economic analysis (TEA) and life cycle assessment (LCA) were conducted. The key reaction mechanism employed is transesterification within PET and PBT chains (Figure (a)).
Waste PET and PBT, which are commonly discarded and structurally well-suited for the designed recycling route, were used as feedstocks. To enhance the sustainability of the process, poly(tetramethylene glycol) (PTMG) was introduced as an additive to promote chain extension and impart biodegradability to the final product.
Process design and comprehensive analysis were conducted for six distinct cases. Commercially available PBT typically contains glass fiber to improve mechanical properties. The presence or removal of glass fiber significantly affects the properties of the final material, allowing for tunable characteristics depending on the separation condition. In addition, varying the PTMG content (20 wt%, 30 wt%, 40 wt%) alters the resulting polymer properties, forming three composition-based cases. Combined with the two glass fiber conditions (removed or retained), a total of six cases were established.
The resulting materials are referred to as RPBRx (Recycled-Polyester based Biodegradable Elastomer), where x indicates the mass fraction of PTMG. If glass fiber is removed from PBT, the material is designated as RPBRx-P; if retained, it is named RPBRx.
The production process of RPBRx-P begins with the dissolution of PBT in hexafluoroisopropanol (HFIP), followed by the removal of undissolved glass fiber. Separation and recovery of glass fiber and HFIP are maximized through centrifugation and filtration, after which the recovered fiber is disposed of. The resulting glass fiber-free PBT is then mixed with PET in an equal mass ratio, along with PTMG at mass fractions of 20 wt%, 30 wt%, or 40 wt%. Under high-temperature and low-pressure conditions (265°C, 0.3 torr), transesterification is catalyzed by titanium(IV) butoxide (TBT), forming the target polymer. During the reaction, PTMG is incorporated into both PET and PBT chains, while ethylene glycol and 1,4-butanediol are released as by-products. These by-products are utilized to supply heat and electricity for the process, and surplus energy is converted into electricity for sale.
In contrast, the production of RPBRx omits the HFIP-based glass fiber removal step, which may result in variations in the final material properties. All process simulations were conducted using Aspen Plus V14. Energy integration was performed via Aspen Energy Analyzer to minimize the overall energy demand of the system (Figure (b)).
Techno-economic analysis (TEA) and life cycle assessment (LCA) were carried out for all six cases. The minimum selling price (MSP) was used as the key indicator for economic evaluation, while multiple environmental impact metrics were considered for the LCA.
The calculated MSPs for RPBR20, RPBR30, and RPBR40 were $1,334/ton, $1,460/ton, and $1,580/ton, respectively. For RPBR20-P, RPBR30-P, and RPBR40-P, the values were higher, at $1,857/ton, $1,879/ton, and $1,895/ton. When compared to current market prices of potential alternatives with similar properties, such as polypropylene (PP) and poly(butylene adipate-co-terephthalate) (PBAT), RPBRx exhibits competitive pricing. Specifically, the market prices of PP and PBAT are reported to range from $1,285–1,800/ton and $2,618–3,367/ton, respectively. While RPBRx falls within this price range, RPBRx-P is priced below that of PBAT, indicating its potential economic advantage despite additional processing.
To ensure a stable supply and consistent quality of raw materials, the procurement of waste PET and waste PBT constitutes the largest portion of the total cost. Compared to conventional plastic upcycling methods, the omission of the decomposition step in this process results in a simplified system, thereby reducing capital investment. As a result, operating costs account for a relatively larger share of the total expenditure, as illustrated in Figure (c). It is evident from the figure that feedstock costs—specifically waste PET and PBT—comprise more than 50% of the overall cost across all cases.
A comparison of the Global Warming Potential (GWP) between the proposed RPBRx and RPBRx-P products and conventional plastics, such as polypropylene (PP) and poly(butylene adipate-co-terephthalate) (PBAT), highlights a significant environmental benefit. While PP and PBAT exhibit GWP values of 2.2 and 12.1, respectively, RPBRx shows substantially lower values of –1.24, –1.16, and –0.30 for x = 20, 30, and 40. Similarly, RPBRx-P records GWP values of –0.15, 0.63, and 1.03 for the same respective PTMG compositions. The negative GWP values primarily stem from the environmental credits associated with recycling waste plastics, which offset the impacts that would have been generated by their disposal.
In addition to GWP, other environmental impact categories also demonstrate lower values for RPBRx and RPBRx-P when compared with PP and PBAT. A comparative assessment was conducted for two representative cases—x = 20 and x = 40—to evaluate the differences in impact profiles (Figure (d)). The analysis reveals that RPBRx-P, in many categories, shows superior environmental performance over its RPBRx counterpart. While RPBRx generally has lower absolute environmental impacts, the relative reduction compared to conventional alternatives is more pronounced for RPBRx-P. This suggests that RPBRx-P may offer greater environmental competitiveness when considered as a market substitute for PBAT.
To further investigate the primary contributors to both cost and environmental impacts, a comprehensive sensitivity analysis was conducted, as shown in Figure (e). This analysis aimed to assess how variations in key parameters influence the overall techno-economic and environmental outcomes of the process. Among the variables examined, the cost of feedstock—namely waste PET and PBT—emerged as the most influential factor. The pronounced sensitivity to feedstock pricing highlights its pivotal role in determining the economic feasibility of the proposed recycling process. This finding underscores the importance of securing cost-effective and stable sources of waste plastic to maintain the competitiveness of the system in real-world applications.
In addition to the sensitivity assessment, Figures (f) and (g) illustrate the cost and environmental impact distributions across individual process subsystems using Sankey diagrams. These visual tools provide a clear and intuitive representation of how resources and burdens are allocated within the process. Specifically, Figure (f) delineates the flow of capital and operating expenditures throughout the system, identifying the unit operations with the highest financial demands. Conversely, Figure (g) focuses on the distribution of environmental loads, including greenhouse gas emissions and other impact categories, across each subsystem. Collectively, these diagrams offer valuable insights into optimization opportunities by pinpointing hotspots where improvements in efficiency or resource use could lead to significant gains in both cost reduction and environmental performance.
This study presents a novel approach to upcycling waste PET and PBT through direct polymer-to-polymer conversion using transesterification reactions with PTMG, aiming to minimize both energy and chemical consumption. By eliminating the decomposition step inherent in conventional chemical recycling, the proposed process achieves economic and environmental advantages, as demonstrated through techno-economic analysis (TEA) and life cycle assessment (LCA). The incorporation of glass fiber removal and PTMG blending allows for the production of biodegradable elastomers (RPBRx and RPBRx-P) with tunable physical properties. Cost analysis reveals that feedstock cost dominates overall expenditure, emphasizing the importance of sourcing strategy. Furthermore, environmental performance, particularly in terms of global warming potential (GWP), shows significant improvement compared to conventional plastics such as PP and PBAT. RPBRx-P, in particular, demonstrates competitive sustainability benefits when considered as a market substitute. These findings suggest that the proposed upcycling strategy offers a viable and scalable pathway toward circular plastic economy initiatives.