2025 AIChE Annual Meeting
(73e) Tuning the Morphological Properties of Granular Hydrogels to Control Lymphatic Capillary Formation
Authors
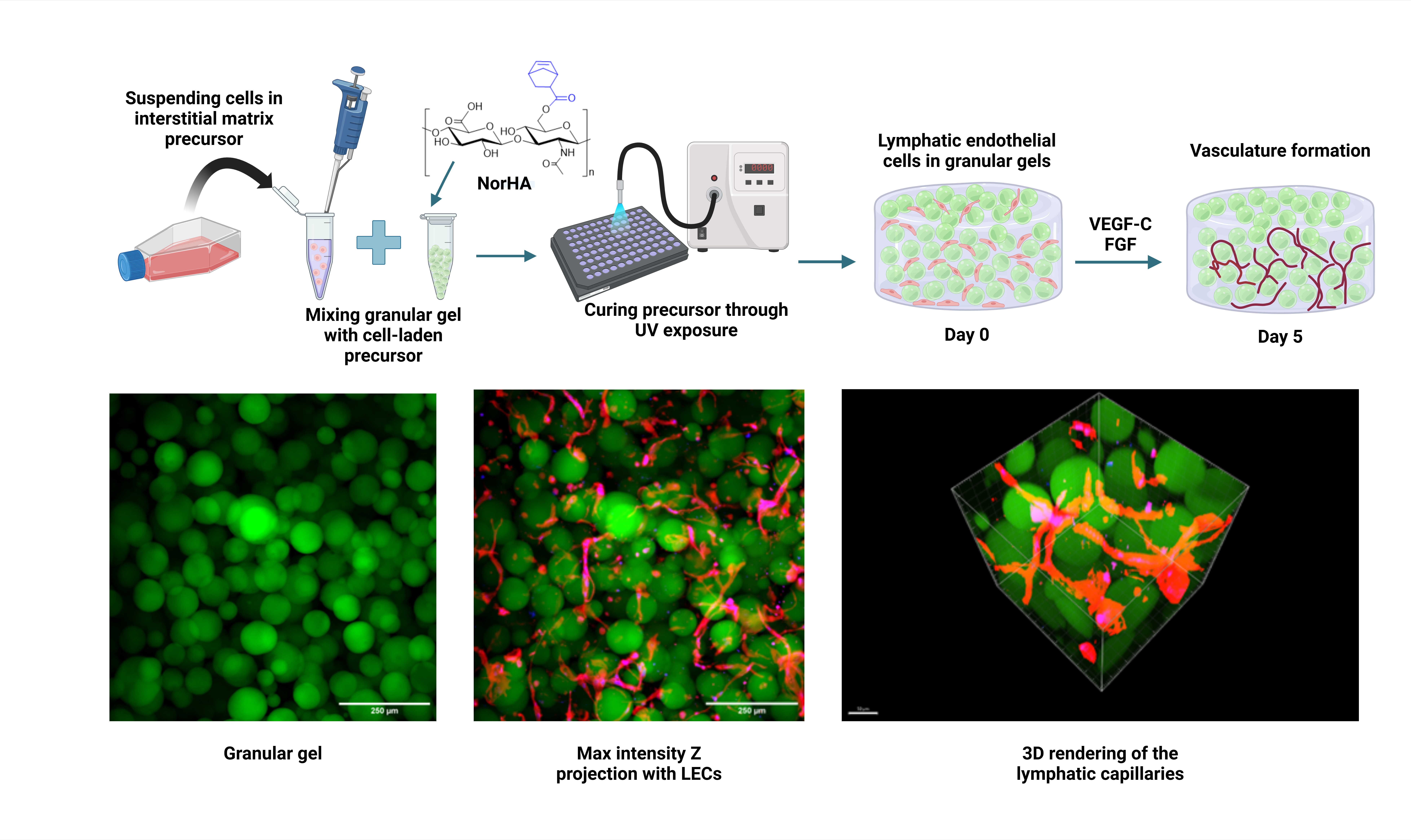
In this study, we investigated the morphological effects of granular gels on the development of lymphatic capillaries. Using norbornene-modified hyaluronic acid (NorHA) polymer and DTT as crosslinker, microgels were fabricated via pipetting and vortexing techniques, yielding distinct morphologies. Granular hydrogels were assembled through centrifugation under loose and tight packing conditions to produce different bulk gel rheological properties and pore morphologies (curvatures). Morphological analysis revealed that vortexing-produced gels exhibited higher porosity, but wider microgel size distribution compared to pipetting, resulting in tighter packing and smaller pores.
Lymphatic capillary formation is only observed under densely packed conditions, when all gels exhibit the same bulk storage and loss modulus, and within a degradable interstitial matrix made with NorHA and an MMP-sensitive crosslinker. The V180s sample exhibited an earlier vessel formation and maturation as supported by key lymphatic gene and protein expressions (LYVE-1, Prox1, PDPN, VEGFR3, Reelin and TIMP-1). For all gels, the internode length of the capillary is a universal 17 microns between branching nodes. However, significantly better connectivity of the lymphatic capillaries was observed for the pipetting granular hydrogel where bigger vessel clusters were formed, with also a lower number of satellite small constructs. The vessel cluster also has a lower curvature for the pipetting template, as the network is not trapped in large voids of the vortexed gel droplets and are able to form linear constructs bridging multiple droplets, when the droplet size is smaller than the universal internode length of 17 microns. This study hence suggests that for granular gels, 3D in vitro lymphatic tube formation is controlled not by the mechanical properties of the gel, but by the pore size and topology (regimented periodicity) of the gel morphology.
Overall, our findings provide novel insights into factors influencing lymphangiogenesis within granular hydrogels, demonstrating the potential to generate lymphatic capillaries without the need for supporting mesodermal cells. By elucidating the morphological effects on lymphatic vasculature development and functionality, our study contributes to the development of platforms for regenerative medicine and modeling of disease progression. These insights pave the way for the design of biomimetic materials capable of promoting lymphatic regeneration and advancing therapeutic strategies for chronic inflammation modulation.