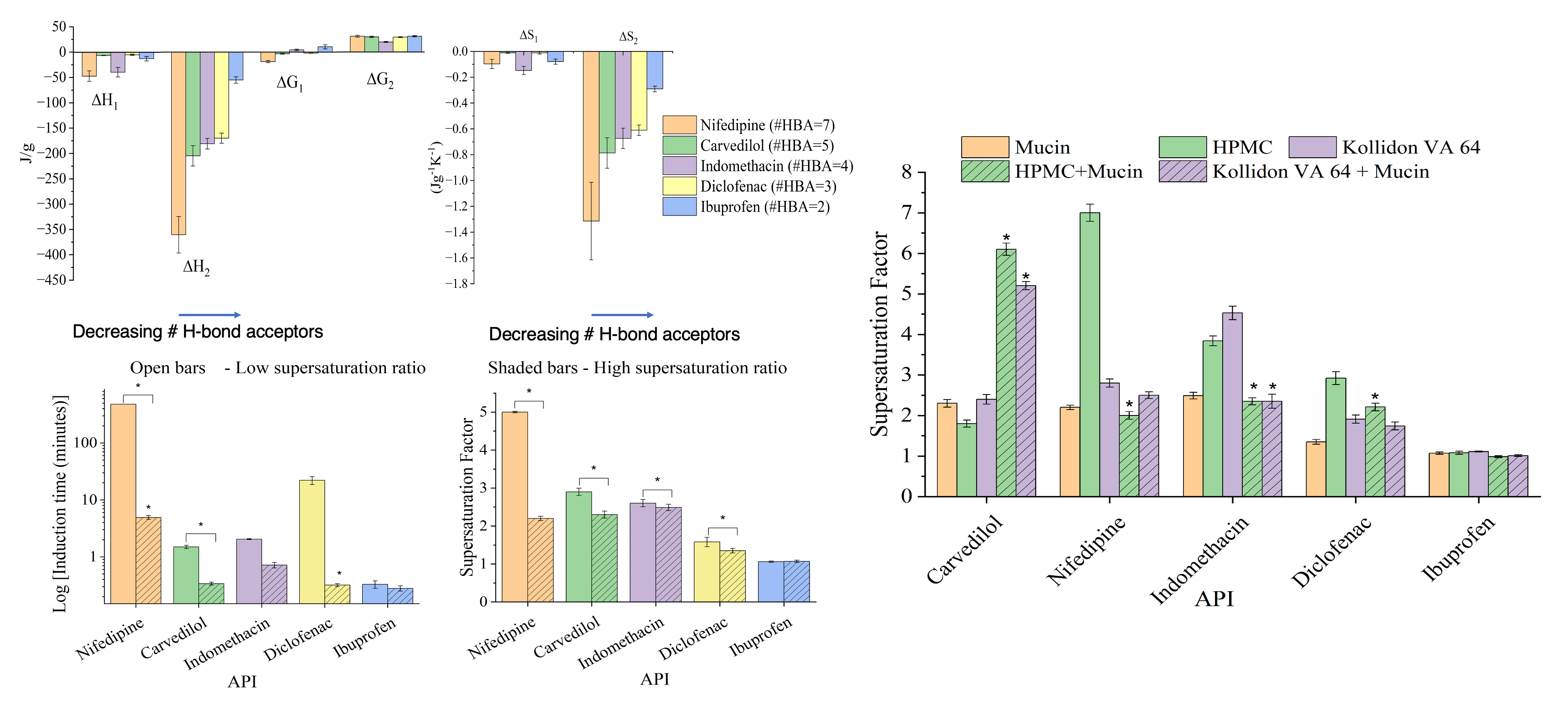
Mucin, the structural component of mucus, is an important component of the intestinal milieu that can undergo numerous types of interactions with supersaturating formulations components, yet biorelevant dissolution testing ignores the role of mucus in formulation performance. Many poorly water-soluble drugs entering clinical trials today rely on amorphous solid dispersions (ASD) which often include polymers such as HPMC and Kollidon® VA 64 to generate and maintain supersaturated drug states to drive absorption. The many functional groups in mucin provides it the opportunity to engage in hydrogen bonding, hydrophobic and electrostatic interactions with the Biopharmaceutical Class (BCS) II drugs upon dissolution of ASD formulations in the gastrointestinal tract. Since ASD polymers stabilize drug supersaturation through mainly through hydrogen bonding with the drugs, we hypothesized that, hydrogen bonding between mucin and drugs can reduce or increase concentration of drug in supersaturated solution in ASD polymers leading to inaccurate evaluation and prediction of formulation performance
in-vivo. To test this hypothesis, we investigated molecular interactions between porcine mucin (III), a reliable model for intestinal mucus, and five BCS II drugs of varied physicochemical properties using isothermal titration calorimetry (ITC) . We then studied the impact of mucin on drug precipitation of the model compounds and compared it with the polymers using high performance liquid chromatography (HPLC). Furthermore, we explored the impact of mucin on the capacity of the ASD polymers to delay drug precipitation
in-vitro.
Thermodynamic modelling and analysis of ITC data suggests that mucin forms strong hydrogen bond and more stable complexes with hydrogen-bond acceptor rich drugs, likely owing to the high number of hydrogen bond donors present in mucin. Not only did the presence of 0.2% (w/v) mucin within the supersaturated environment significantly reduced drug precipitation rates comparable to polymer precipitation inhibitors, but also altered the precipitation inhibitory effects of HPMC and Kollidon® VA 64. The stability of the drug-mucin complex correlates positively with the precipitation inhibition potential of mucin, suggesting that mucin stabilizes drug supersaturation by forming strong hydrogen bonds and stable drug-mucin complexes.
These findings highlight the need to incorporate mucus activity into biorelevant dissolution testing to better predict in vivo drug precipitation risks. Modifying testing methodologies to include appropriate mucus activity could improve the accuracy of drug supersaturation and precipitation assessments, ultimately aiding the development of optimized drug delivery systems.