2025 AIChE Annual Meeting
(191q) Syringability and Physical Stability of Pre-Filled Intra-Mammary Injectors
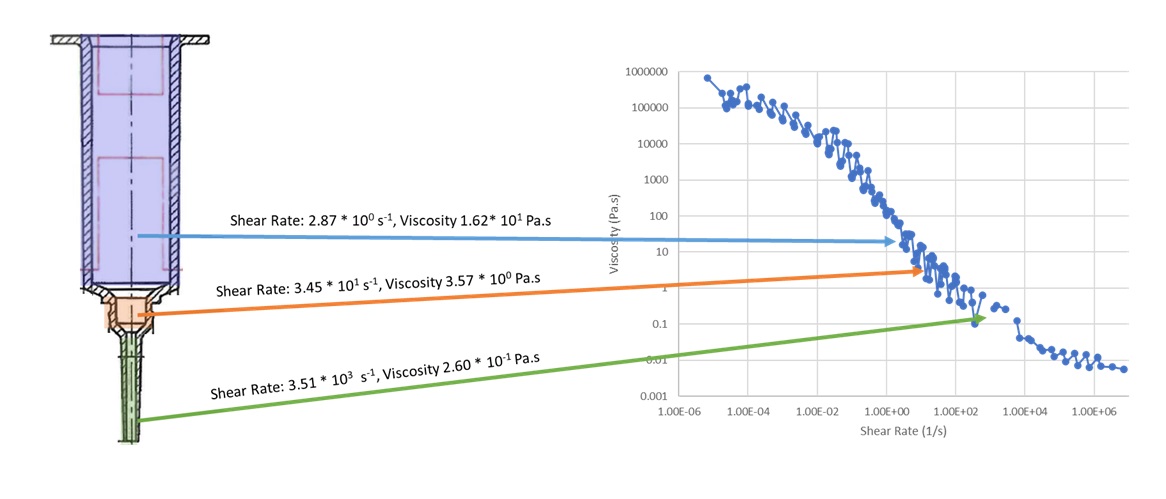
Bovine mastitis has long been one of the leading disease issues faced by dairy farmers around the world. One leading treatment method for this disease is pre-filled intra-mammary injectors. These injectors are typically filled with a suspension of one or multiple antibiotic active pharmaceutical ingredients (APIs) in an oily medium such as peanut or sesame oil. Formulations must balance the need to suspend the API long term to prevent settling and syringe tip clogging with the need to be easily syringed out by farmers. Ease of syringing is especially important especially important, considering that multiple cows may be treated in a single day, each cow can have up to 4 teats treated, and each dose is 3g per infected teat. To meet these two competing needs, surfactants and waxes are added to the formulation to give the product shear thinning characteristics. In this poster, the results of statistical modeling of a series of common excipients at various levels will be discussed. Settling rate of these suspensions was characterized using a LUMiSizer Dispersion Analyzer to measure the velocity of suspended particles under accelerated gravity conditions. The syringability was characterized using a texture analyzer to measure the force necessary to eject the formulation through the syringe tip. Finally, rheological measurements were used to characterize the viscosity of the formulation in the portions of the syringe with different diameters (see image). Experimentally, the most critical factor across these experiments was the level and type of wax that was used. This factor increased physical stability of the formulation but did also increase the amount of force needed to expel the contents of the syringe. Finally, viscosity characterizations were used to compare formulations to a benchmark formulation that is no longer on the market to show similar viscous behavior across a wide shear rate range.