2025 AIChE Annual Meeting
(149e) Separation of Fly Ash Cenosphere from Fly Ash Using Hydrocyclone Separator and Its Application in Dye Removal
Authors
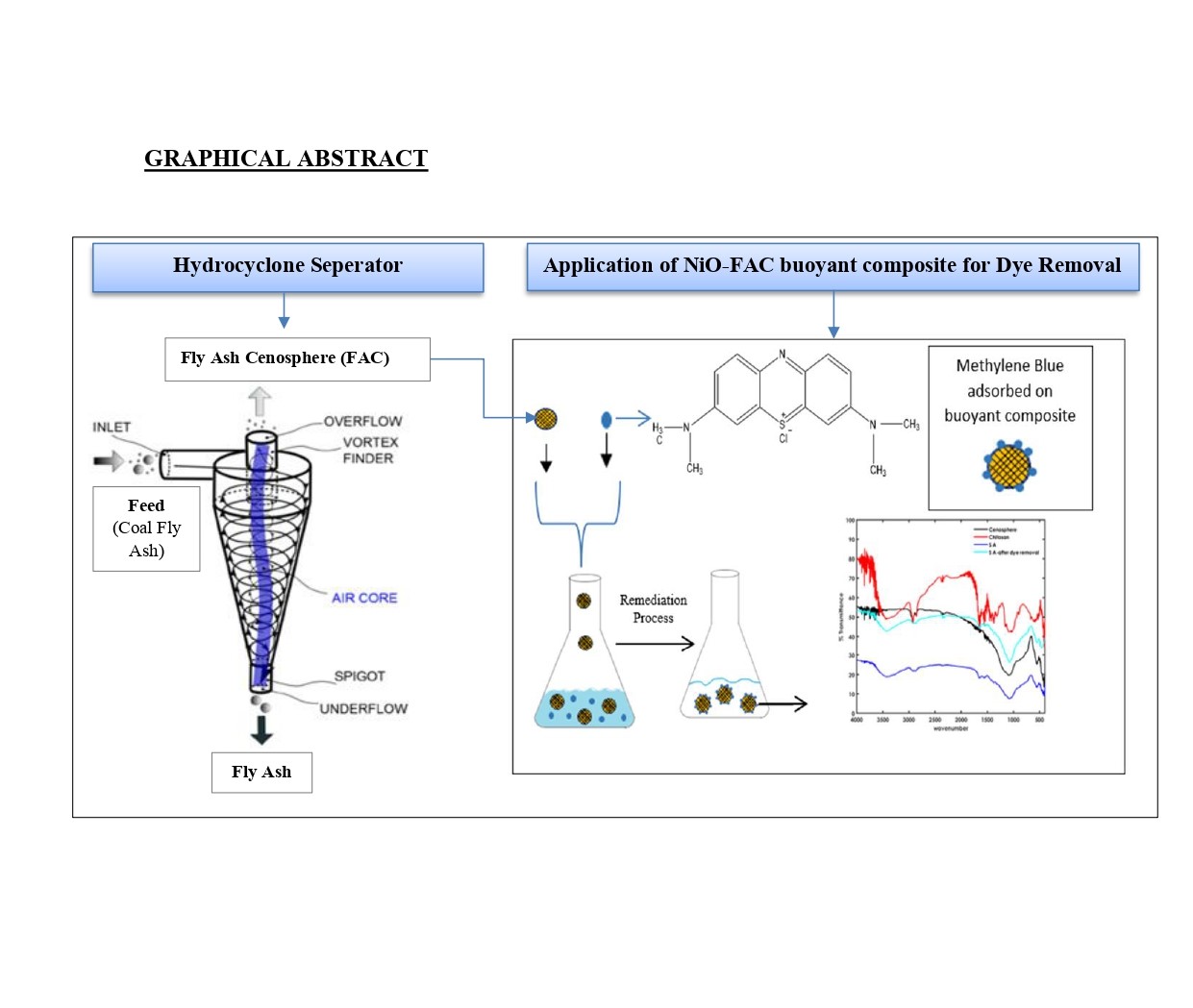
The first objective emphasizes the separation of FACs from coal fly ash using a specially designed double-inlet hydrocyclone separator. Fly ash slurry was introduced at pressures of 1, 2, and 3 bar, where lighter cenospheres migrated towards the central axis, forming the overflow product, and heavier particles moved to the periphery, discharged as the underflow. The study found that increasing the pressure improved the yield of the overflow product, concentrating more cenospheres. The separation process achieved cenospheres recovery rate of 85% with underflow product yield of 84%, aligning with trends observed in dry separation methods like micron separators. Notably, Newton's efficiency correlated with the yield of the underflow product, peaking at an optimum level of 0.65 before declining. These results demonstrate the hydrocyclone's efficiency in recovering cenospheres while providing valuable insights into optimizing operating conditions for industrial applications.
The second objective explored the application of NiO-coated chitosan-cenosphere composites for the adsorption of methylene blue (MB) dye, a common organic pollutant in wastewater. FACs, being lightweight and having a high surface area-to-volume ratio, were chosen as the base material. The cenospheres were coated with chitosan and NiO through a hydrothermal process, with silane coupling agents and epichlorohydrin as cross-linking reagents. Morphological analysis via scanning electron microscopy (SEM) confirmed the composite's hollow structure, while Brunauer–Emmett–Teller (BET) analysis revealed a surface area of 8.73 m²/g and a pore volume of 2.05 cm³/g. Fourier-transform infrared (FTIR) analysis identified functional groups such as NH₂, OH, and CO, which enhance adsorption capabilities. The hollow FAC exhibited an average particle size of 9.6 µm, with SiO₂ and NiO content increasing to 64.946% and 3.54%, respectively, after activation and coating. Batch adsorption experiments assessed the composite's performance as an adsorbent, for removal of Methylene Blue exploring the effects of pH (2–12), initial dye concentration (50–200 mg/L), temperature (37–47°C), and contact time (0–24 hours).
The composites demonstrated effective adsorption of MB, achieving maximum dye removal efficiency of 83% under optimal conditions: a pH of 7.6, a temperature of 37°C, a dye concentration of 50 mg/L, and a 10-hour equilibrium time. The pseudo-second-order model for adsorption kinetics showed that chemisorption was the dominant mechanism. Furthermore, thermodynamic analysis revealed that the adsorption was spontaneous and exothermic, with a ΔH value of -35.56 kJ/mol and a ΔS value of -98.23 J/mol·K. The buoyant nature of the composites, combined with the catalytic properties of NiO, enhances their potential for efficient dye removal from water.
In conclusion, this study showcases the versatility of fly ash cenospheres as a valuable resource for industrial and environmental applications. The hydrocyclone separator effectively recovered FACs, which were subsequently used as substrates for advanced coating and composite synthesis. The NiO-coated chitosan composite demonstrated potential as an eco-friendly and effective material for wastewater treatment due to its adsorption capabilities.
These findings highlight the potential of FACs to address critical challenges in material science and environmental sustainability.