2025 AIChE Annual Meeting
(191ap) Scalable Microfluidic Production of Well-Controlled Micro-Drug Delivery Systems
Authors
In this work, droplet-based microfluidic devices (flow-focusing) are used to synthesize Poly(lactic-co-glycolic-acid) (PLGA) microparticles for drug (i.e, Levosimendan) delivery. The system was optimized to accurately control the final particle size, uniformity, drug-loading efficiency, and drug-release profile by manipulating operating parameters (i.e., flow rate ratio of the two phases, surfactant and polymer concentration). The process was performed at two distinct scales (100 & 30 μm ID) to assess the system’s scalability. Flow pattern characteristics (e.g., droplet size) were captured using high-speed imaging, whilst Scanning Electron Microscopy (SEM) and UV/vis spectrometry were applied to characterise microparticle morphology and evaluate drug release profiles. Computational Fluid Dynamics (CFD) simulations were performed to reveal complex flow patterns (i.e. internal circulations) and predict droplet formation dynamics.
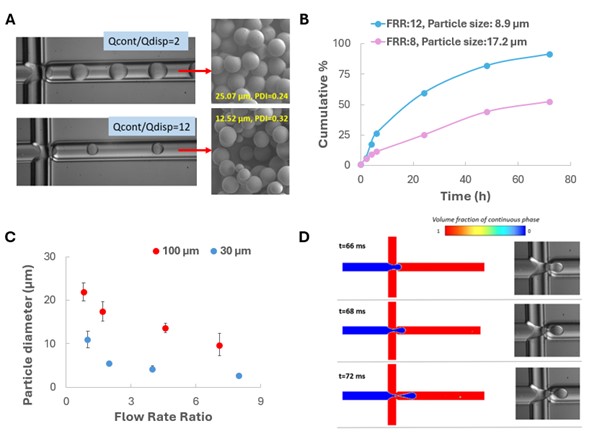
Controllable synthesis of microparticles (1–25 μm) was achieved with high uniformity (PDI ~0.3) and an average encapsulation efficiency of ~48%. Droplet sizes ranged from 5.6 to 110 μm. The flow rate ratio was identified as the dominant factor influencing droplet and particle sizes (Figure 1A), with increased ratios leading to smaller sizes due to enhanced shear forces and supersaturation. This trend also correlated with faster drug release (Figure 1B), attributed to the higher surface area-to-volume ratio of smaller particles. Scaling down to a 30 μm channel yielded a threefold reduction in droplet and particle size (Figure 1C), enhancing mass transfer but slightly limiting throughput. Nevertheless, particle productivity in the 30 μm device remained nearly constant, with an average production rate of 9.4E-04 (±3.75E-05) g/h. CFD simulations were in good agreement with experimental results (10% max deviation) and were successfully implemented to analyse the particle formation process and predict droplet formation (Figure 1D).
This study demonstrates how tuning operating conditions enables precise control over particle features and drug release profiles. CFD-assisted design supports the optimization and scalability of these systems, paving the way toward industrial translation.
Figure 1: (A) Effect of flow rate ratio (FRR=Qcont/Qdisp) on the particle size and PDI visualised by high-speed camera images of the droplet formation for Qcont/Qdisp 2 and 12 and respective SEM images of the microparticles synthesized, (B) Release profiles of drug Levosimendan, from PLGA microparticles of two different sizes, resulting from different values of flow rate ratios. The microparticles were synthesized in 100μm ID flow-focusing microdevice, (C) Effect of flow rate ratio on the particle diameter at two geometrically similar flow-focusing devices of 100 μm and 30 μm ID. The flow rate of the dispersed phase (Qdisp) remained constant at 0.7ml/h (for the 100μm ID microdevice) and at 0.3ml/h (for the 30μm ID microdevice), (D) CFD images of droplet formation mechanism in 100 μm flow focusing device under FRR=10, Qdisp=0.7 ml/hr, Ca=0.021, Re~14 (dripping regime). Colours represent the volume fraction of the continuous phase, where blue: volume fraction=0 and red: volume fraction=1.
References
- Vargason et al., (2021) Nat Biomed Eng 5, 951–967
- Tomeh M.A. et al. Mol. Pharmaceutics 2020; 17:p. 4421-4434