2025 AIChE Annual Meeting
(720h) Revealing the Hydration-Mediated Direct Vapor Pathway for Simultaneous Water Splitting and Hydrogen Liberation from LiAlH4
Authors
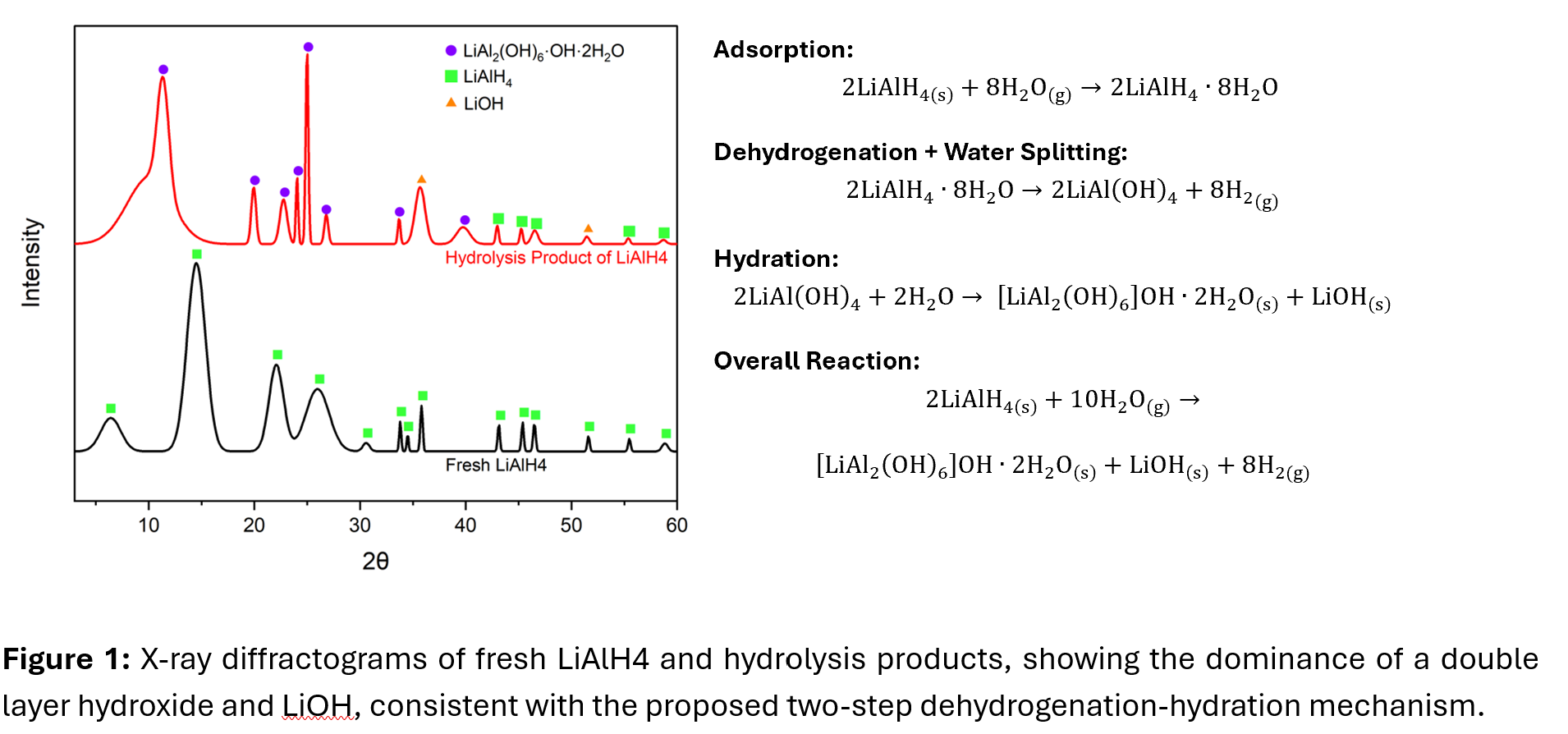
First, the results reveal that for the reaction to reach completion, excess water vapor relative to stoichiometric amount is required. Ex situ X-ray Diffraction (XRD) shows that solid products from LiAlH4 hydrolysis are predominantly lithium-aluminum Layer Double Hydroxides (Li-Al LDH) with the chemical formula of LiAl2(OH)6.OH.2H2O and LiOH.
Next, kinetic evaluation of powder hydrolysis shows an induction period for water uptake followed by hydrogen release, which when combined with in situ Fourier transformed diffuse reflectance spectroscopy (DRIFTS), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and thermogravimetric analysis (TGA) supports a new mechanism for Li-Al LDH formation from LiAlH4 vapor hydrolysis. This new pathway proceeds by direct interfacial surface rearrangement and is dissimilar to conventional routes which follow dissolution-precipitation or salt imbibition (intercalation).
By observing O-H bending vibrations at 1400-1500 cm-1, O-H stretching vibration at 3540-3550 cm-1, the DRIFTS data was used to track how surface chemical changes influence the evolution of the bulk phase.
This new understanding is essential for identifying conditions for maximal, controlled, on-demand H2 production from solid storage materials to power the next generation of long-range transportation.