2025 AIChE Annual Meeting
(73a) Reconfigurable Organ Chip for Investigating the Importance of Aortic Smooth Muscle Cells in the Nerve-Artery Environment
Authors
Organ chip technology has immense potential to advance drug screening, diagnostics, and tissue engineering research. Because they allow control over spatial configuration of multiple cell types, organ chips provide increased relevance to the human physiology compared to traditional cell culture platforms and animal models. Furthermore, design-driven organ chip development allows monitoring of cell-cell interactions and maturation of human cells over time.
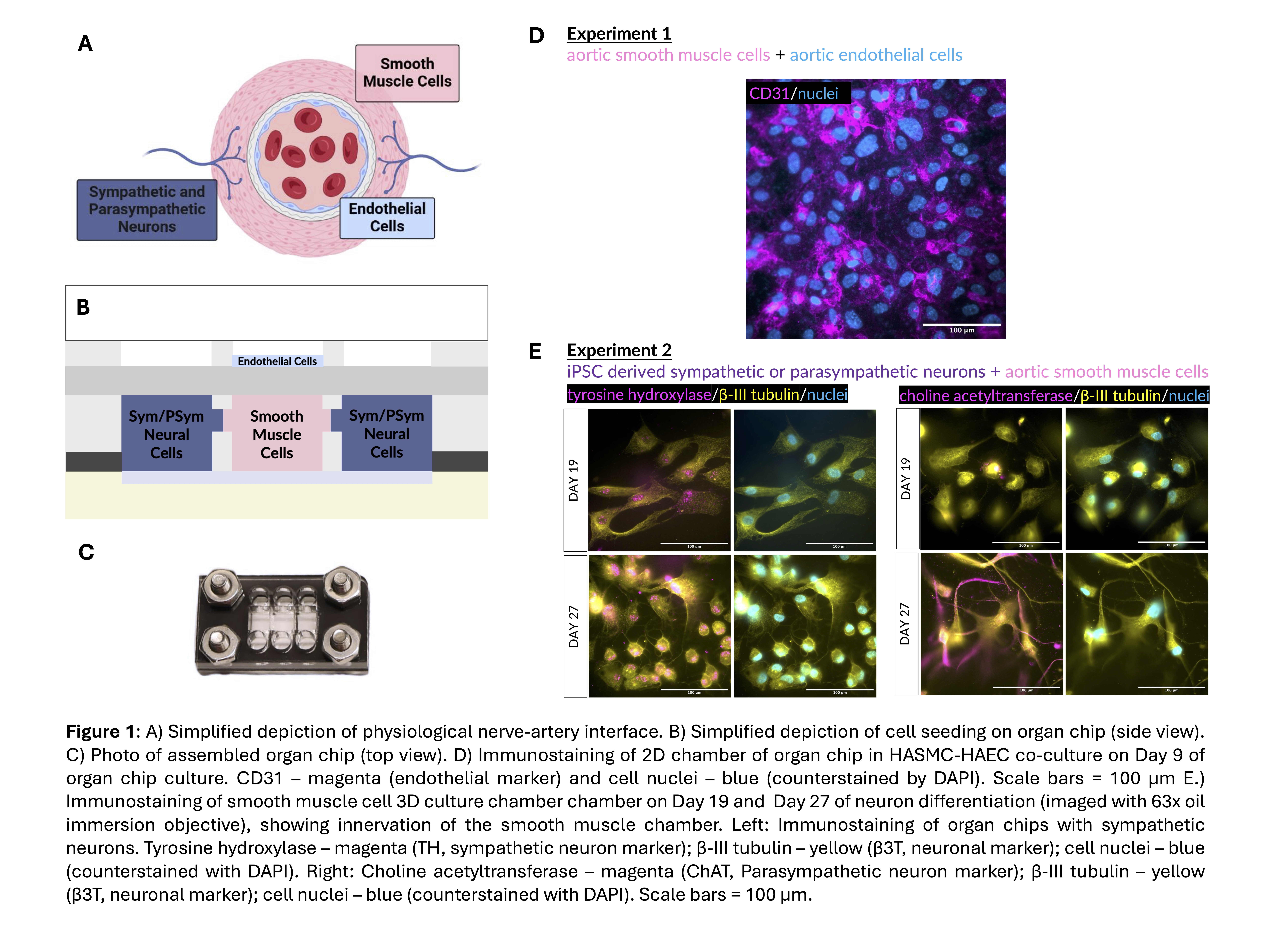
Despite recent advances, several hurdles prevent the widespread adoption of organ chips for biological studies. High fabrication costs and wait times can be inhibitive, and maintenance of sensitive human cell types, especially those that are post-mitotic in their maturity (e.g. neurons), is also challenging. Here we present a novel, reconfigurable organ chip to address these concerns. Our organ chip is the first of its kind to include 2D and 3D culture layers that can be separated from each other for biological assays, which expands experimental possibilities compared to other organ chips. Additionally, compared to organ chips with culture chambers connected by microchannels, our system with GelPins allows the advantage of multiple interfacing 3D cultures as well as contact with 2D cultures. To demonstrate the utility of our platform for tissue engineering vascularization and innervation, we chose to model an artery as well as the nerve-artery system, where vascular and nerve cells interface physiologically (Figure 1A). To do so, we co-cultured gelatin methacryloyl (gelMA)-encapsulated human aortic smooth muscle cells with human aortic endothelial cells to model the aortic environment. We then modeled the human nerve-artery interface by co-culturing gelMA-encapsulated human aortic smooth muscle cells with gelMA-encapsulated differentiating neural cells (derived from human induced pluripotent stem cells, iPSC), which highlights the potential of the organ chip to support sensitive innervated and vascularized human cell cultures (Figure 1B). Additionally, we utilized the reconfigurability of our organ chip to stain and image organ chip culture layers separately, demonstrating the use of our platform for biological assays. To our knowledge, our organ chip is the first humanized nerve-artery model to include an organ chip or a 3D culture.
Methods
The organ chip includes three layers: a 3D cell culture layer, a 2D cell culture layer, and a media reservoir layer. The 3D cell culture layer allows the contact of adjacent hydrogel culture chambers through GelPins (previously established by our lab), forming a contiguous culture, and has a glass bottom to facilitate high resolution microscopy. Above the 3D cell culture layer is the 2D cell culture layer, where cells are seeded onto a semi-permeable membrane, allowing contact with the hydrogel-encapsulated cells below. The media layer is added at the top of the assembly. The organ chip is fabricated using polymethyl methacrylate (PMMA), polyethylene terephthalate (PET), Viton, and resin pieces which are laser cut or 3D-printed. The three chip layers are formed by assembling the fabricated pieces layer-by-layer with double sided tape, as previously established by our lab. The three layers are then stacked and held together using stainless-steel screws and nuts (Figure 1C). Use of widely available and inexpensive materials (~10 USD per chip vs 150-500 USD per design) and a rapid fabrication method (hours vs. weeks) presents several advantages over traditional stereolithography-fabricated polydimethylsiloxane (PDMS) organ chips, including more suitable material compatibility for drug testing as well as increased versatility by readily making design adjustments as needed. Additionally, the three layers of our organ chip can easily be removed from each other by disassembling the hardware and gently pulling the layers apart, allowing direct access to each individual cell type for assays.
After device fabrication and sterilization, we conducted arterial and nerve-artery co-cultures on our organ chip. For the arterial organ chip, gelMA precursor solution was formed by diluting gelMA (synthesized from fish gelatin) to 5% w/v in endothelial medium with 0.5% w/v lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP). On Day 0 of culture, 10 μL of gelMA precursor solution was added to the middle 2D chamber and crosslinked, to coat the chamber. Human aortic smooth muscle cells (HASMC) were then resuspended in the gelMA precusor solution. The HAMSC/gelMA precursor solution was seeded into the middle 3D culture chamber of the organ chip and crosslinked with blue light. On Day 5 of organ chip culture, human aortic endothelial cells (HAEC) were seeded onto the middle 2D chamber. The arterial organ chip was cultured for a total of 9 days in Bovine Brain Extract-containing endothelial medium.
To form the nerve-artery organ chip, Day 6 neural progenitor cells (NPC, derived from human iPSC) and HAMSC were each resuspended in gelMA precursor solution (5% w/v gelMA, 0.5% w/v LAP). HASMC/gelMA precusor solution was seeded into the middle 3D culture chamber of the organ chip and crosslinked with blue light, then NPC/gelMA precusor solution was seeded into each outer 3D culture chamber of the organ chip and crosslinked with blue light. Nerve-artery organ chips were maintained in 1:1 neural differentiation medium: endothelial medium containing vascular endothelial growth factor (VEGF). We directed the neural cells towards either sympathetic or parasympathetic differentiation at the maturation stage. Neurovascular organ chips were maintained for up to 27 days of neural differentiation.
At various culture endpoints, the organ chips were disassembled, fixed, and immunostained. The 2D layer of the arterial chip was stained for CD31 (or PECAM-1, endothelial marker), while the 3D hydrogel layer was stained for smooth muscle markers: α-smooth muscle actin (α-SMA) and smooth muscle myosin heavy chain (SM-MHC). The 3D layer of the nerve-artery chip was stained for ß-III-tubulin (ß3T, neural marker) and tyrosine hydroxylase (TH, sympathetic neuron marker) or choline acetyltransferase (ChAT, choline acetyltransferase). Samples were imaged using an inverted fluorescence microscope (Zeiss Axio Observer).
Results and Significance
We developed an organ chip with interfacing cell culture layers that can be separated from each other, allowing access to each cell culture chamber for analyses. We were able to co-culture smooth muscle cells to model the arterial and nerve-artery environment, which involved co-cultures spanning the 2D and 3D culture layers of the chip as well as differentiation of human iPSC-derived neural cells on chip. Utilizing the reconfigurability of our organ chip, we were able to disassemble, stain, and image the 2D and 3D layers of our organ chip separately. As the 3D cell culture layer can be easily maneuvered after removal from the overall chip assembly, we were able to image with 63x oil immersion lens, which is not possible for most other organ chip platforms (Figure 1D-E).
In our arterial chip, we co-cultured endothelial cells in the 2D culture layer and smooth muscle cells in the 3D culture layer of our chip, stained the two layers separately, and visualized both endothelial and smooth muscle markers in the respective chambers. In our nerve-artery chip, we confirmed that the neural cells migrated into and innervated the adjacent smooth muscle chamber by the presence of ß3T in that chamber. This finding may be reflective of physiological interactions between the immature cell types. We also visualized the presence of TH and ChAT in the sympathetic and parasympathetic differentiation groups, respectively, by imaging these transient catalysts with a 63x oil immersion lens. Furthermore, our platform was able to support culture of sympathetic neural cells for up to Day 27 of differentiation, as shown by differentiation Day 27 stains of TH and ChAT. Overall, in this study we established the utility of our organ chip for long-term vascularized and innervated cultures of human cells.
Though we focused specifically on vascular and neural cells in this study, our findings demonstrate that our organ chip platform has potential for further application, especially in modeling other vascularized and innervated systems. Our approach of layer-by-layer assembly of laser cut and 3D printed pieces is a rapid and inexpensive method that can be applied to other platforms. The unique separable layers of our organ chip facilitated multi-channel imaging and could be very useful in other co-culture systems as well. In future work, we aim to continue exploring these applications by conducting further experiments to understand the importance of smooth muscle cells in the nerve-artery environment, modeling other vascularized and innervated multi-organ systems, and conducting multiplexed imaging experiments.