2025 AIChE Annual Meeting
(74b) Producing Eicosapentaenoic Acid from Waste Cooking Oil Using Yarrowia Lipolytica
Authors
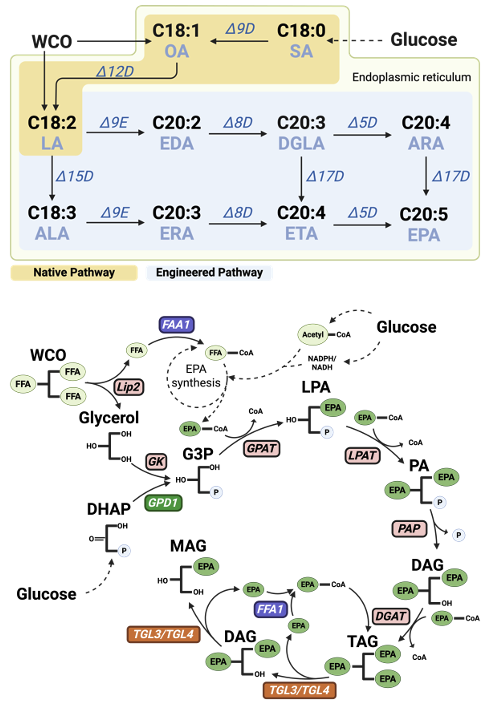
Utilizing the unconventional yeast Yarrowia lipolytica as a cell factory presents a feasible option for the biomanufacturing of EPA. Although producing EPA with Y. lipolytica offers a more consistent and eco-friendly method, yielding high-quality and safer EPA compared to traditional fish oil manufacturing, the yield remains low when glucose is used as the carbon source. Globally, it is estimated that more than 15 million tons of waste cooking oil (WCO) are generated annually. As a Generally Recognized as Safe (GRAS) microorganism, Y. lipolytica can efficiently utilize lipids as a carbon source. Since the major components of WCO are oleic acid and linoleic acid, using WCO as a carbon source not only addresses potential pollution caused by improper disposal of waste cooking oil but also shortens the metabolic pathway for EPA synthesis from the carbon source.
In our research, we investigated and optimized the engineering of a Y. lipolytica strain for efficient conversion of WCO into high-value EPA. Our strategies included: (1) preventing triglycerides (TG) from converting back into free fatty acids (FFA), thus enhancing cell viability and reducing EPA leakage from cells; (2) developing a strong dynamic control promoter for EPA synthesis to reduce metabolic burden and ensure high-efficiency expression of key enzymes for converting WCO to EPA; (3) specifically optimizing the metabolic pathway for converting WCO to EPA; and (4) improving the efficiency of desaturase enzymes by relocating key enzymes to specific subcellular locations within Y. lipolytica, thereby enhancing the utilization of reducing power and further boosting EPA synthesis. By replacing glucose with WCO as a carbon source, we achieved a more than 50% improvement in EPA biomanufacturing titers. To the best of our knowledge, our study is the first to demonstrate a direct and highly efficient microbial process for converting waste cooking oil into a valuable omega-3 oil with significantly increased titers and yields.