2025 AIChE Annual Meeting
(239f) Plasmid DNA Vaccine for Post-Surgical Immunotherapy Against Murine Melanoma
Authors
Methylated (MpDNA) or unmethylated (UMpDNA) plasmids expressing the model antigen ovalbumin (OVA) were administered intradermally and subcutaneously proximally to 5 mm diameter full-thickness wounds made in the dorsum of C57BL/6 mice. Wound closure was tracked for 7 days, and submandibular bleeds were carried out on day 13 to determine anti-OVA antibody levels. A booster dose of UMpDNA or MpDNA was administered on day 14, and the mice were challenged with OVA-expressing (B16F10-OVA) or non-OVA expressing (B16F10) murine melanoma cells. Tumor growth and mice weight were tracked till the endpoint (any ulceration or tumor volume >1.5 cm3) or day 90 post tumor challenge. Terminal cardiac bleeds, to determine the levels of the anti-OVA antibodies, and end organs for histology were also collected.
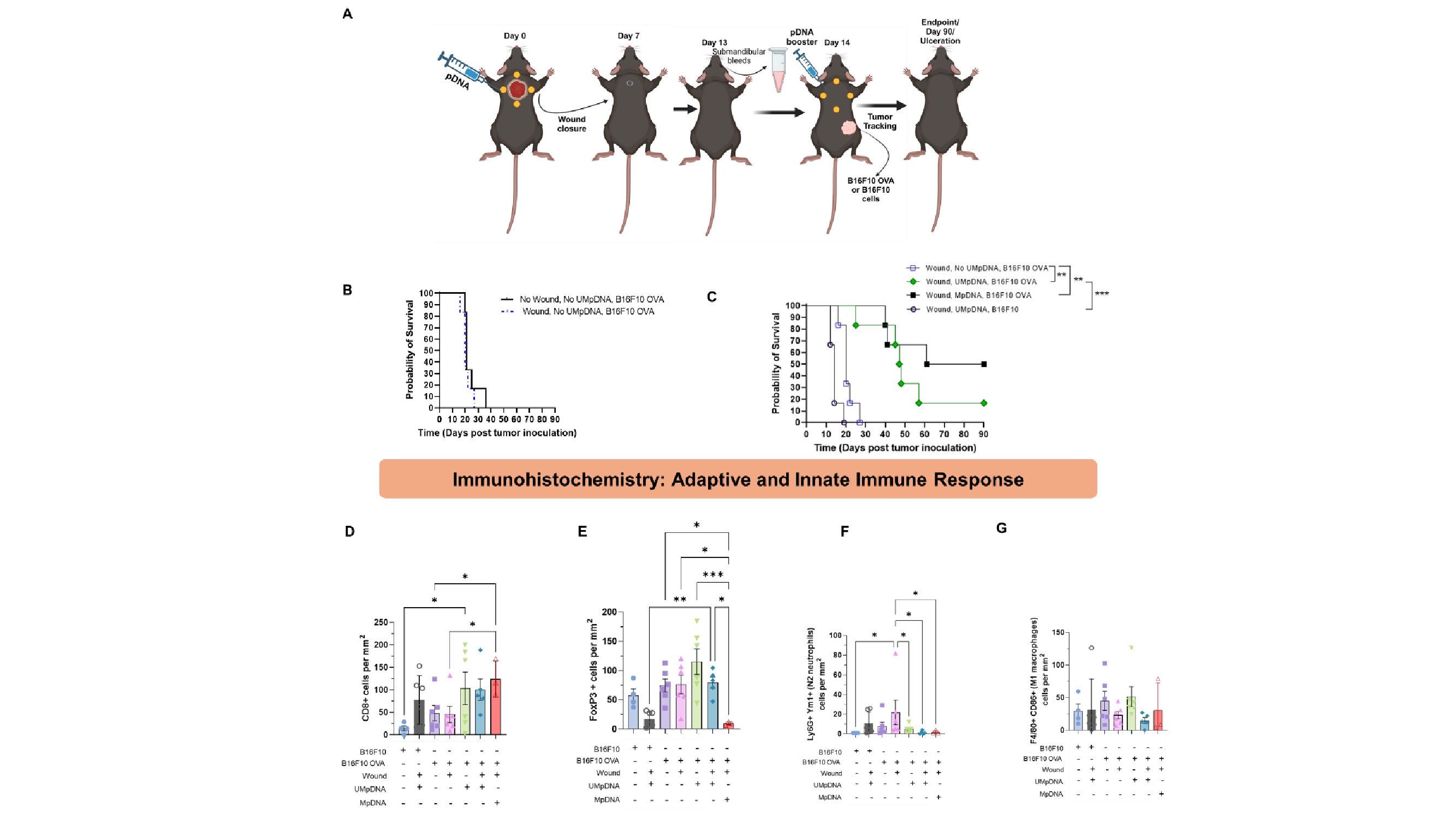
From our wounding study, we observed that UMpDNA-OVA facilitated in 60% wound closure compared to MpDNA-OVA by day 7. From the tumor growth statistics, we noticed that mice challenged with B16F10, i.e. those lacking expression of OVA, were dead by day 20, indicating the need for antigen expression in tumors. A similar trend was observed in mice challenged with B16F10-OVA cells in absence of OVA-expressing UMpDNA, indicating the need for the cancer vaccine. A boost in survival was observed in mice treated with OVA-UMpDNA in absence of wounding with mice surviving for 70 days. Wounded mice treated with OVA-UMpDNA or OVA-MpDNA treatment showed significantly slower tumor progression, resulting in the survival of 1 out of 6 mice for UMpDNA and 3 out of 6 mice for MpDNA treatments till day 90 without signs of tumor development. IgG1 levels were significantly higher in pDNA treatment groups than non-pDNA treated groups. Immunohistochemical analyses indicated high infiltration of CD8+ T-cells and low levels of CD4+ T-cells and FoxP3+ (regulatory) T cells in MpDNA-treated mice, whereas UMpDNA treatment showed elevated levels of CD8+ T-cells, CD4+ T-cells, FoxP3+ (regulatory) T-cells, indicating a comprehensive immune system activation, which also correlated with better wound closure efficacy with these.
CD19+ B cell immune cell infiltration levels were high in UMpDNA treatment compared to MpDNA. UMpDNA treatment also resulted in highest diameter of vasculature indicating a significant effect on structural angiogenesis. Low infiltration of pro-resolution macrophages and neutrophils were seen in both UMpDNA and MpDNA treatment groups.
Plasmid DNA in which prokaryotic sites were unmethylated facilitated closure of full thickness wounds and improved survival in tumor-challenged mice. A greater survival benefit was seen with antigen-expressing methylated DNA.
Figure 1: A. Schematics showing the timeline of wounding and tumor cells inoculation in C57BL6/J mice. (B,C) Kaplan-Meier statistics showing survival rates of mice in UMpDNA-OVA, Wounded mice compared to other groups. (D,E) Adaptive immune response markers (CD8 and FoxP3) and innate immune response markers (F4/80, CD86, Ym1 and Ly6G)