2025 AIChE Annual Meeting
(149b) Multifunctional Metal Oxide Membrane for Simultaneous Oil-Water Separation and Contaminant Degradation
Authors
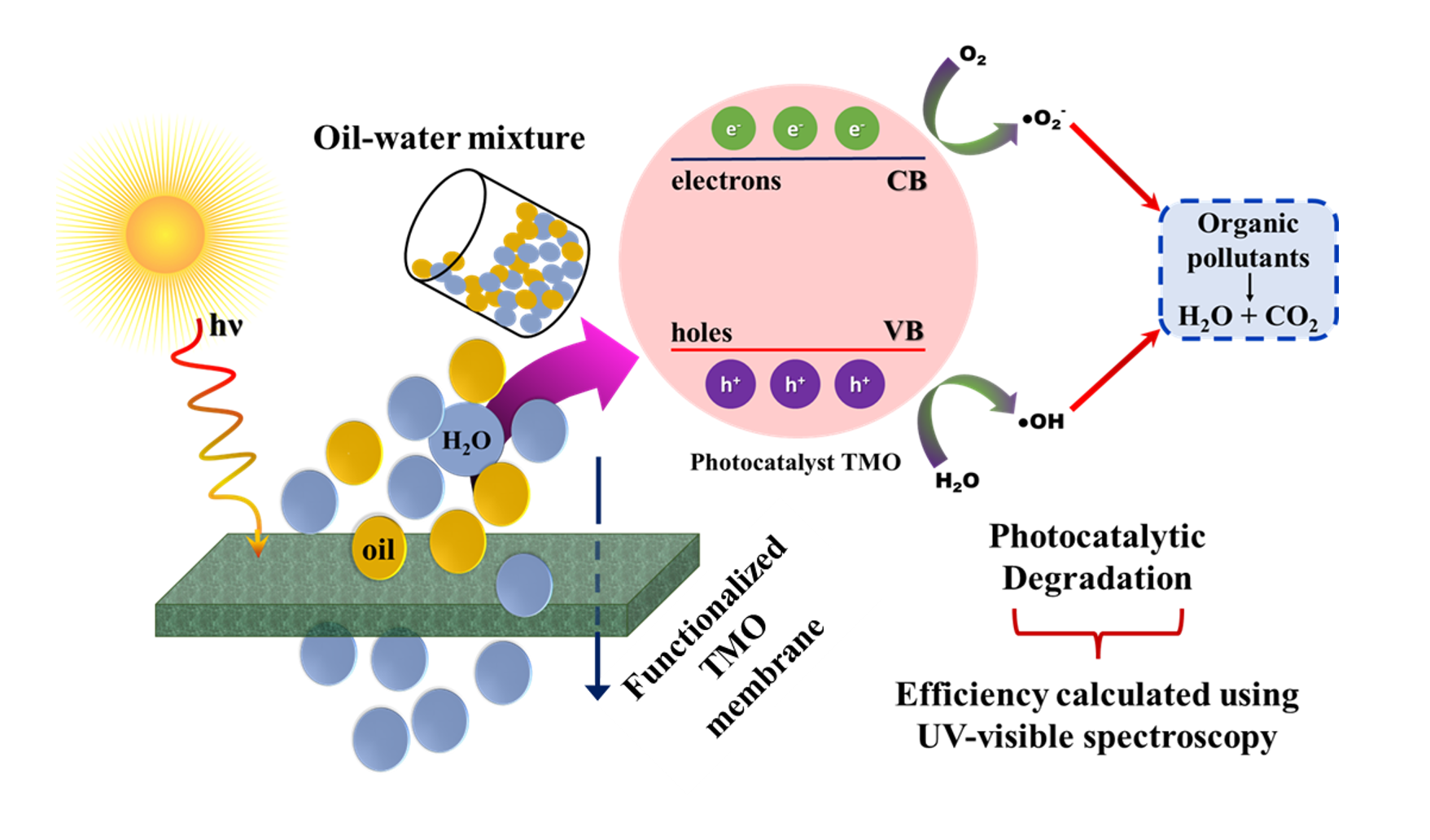
Nickel titanate (NTO) synthesized via a polymer precursor-based sol-gel route using Styrene Acrylonitrile (SAN), has been known to possess multiple band gap values (2.25–3.47 eV) allowing efficient absorption of both visible and UV spectral regions of the solar spectrum [1]. In the present work, photocatalysis experiments performed using NTO nano powders as catalysts showed 76.2% degradation efficiency of methylene blue dye in water, which is comparable to that of TiO₂ (81%), the most commonly used photocatalytic material. It is further planned to fabricate multipurpose membranes of these nano powders for combined oil-water separation and photocatalytic purification applications. The fabrication will be done through a colloidal lithography route to achieve microscale topographic texturing [2]. For this purpose, specific textures present in superhydrophobic species like Lotus leaves and Periwinkle petals were replicated onto polydimethylsiloxane (PDMS) moulds. The resulting PDMS moulds showed enhanced hydrophobicity (contact angles in the range 130° -145°), attributed to the surface roughness, governed by Wenzel’s and Cassie-Baxter’s theories. It is planned to cast NTO-binder mixtures onto these PDMS templates followed by subsequent sintering at a suitable temperature to fabricate porous membranes. These findings and the proposed work highlight the potential of NTO-based materials for simultaneous separation and degradation for the treatment of oil-contaminated water, compared to currently known solutions for this problem.
References
[1] Kumar, B. S., Shanmugharaj, A. M., Kalpathy, S. K., and Anandhan, S. (2017). Ceramics International, 43(9), 6845-6857.
[2] S. Mattaparthi, D. Sablaniya, S. Rajendran, A. K. Singh, S. K. Kalpathy, and S. Rowthu. (2022). Colloids and Surfaces A: Physicochemical and Engineering Aspects, 647, 129112.