2025 AIChE Annual Meeting
(653e) Modulating Cellular Response through Biophysical Properties of Bacterial Extracellular Vesicles
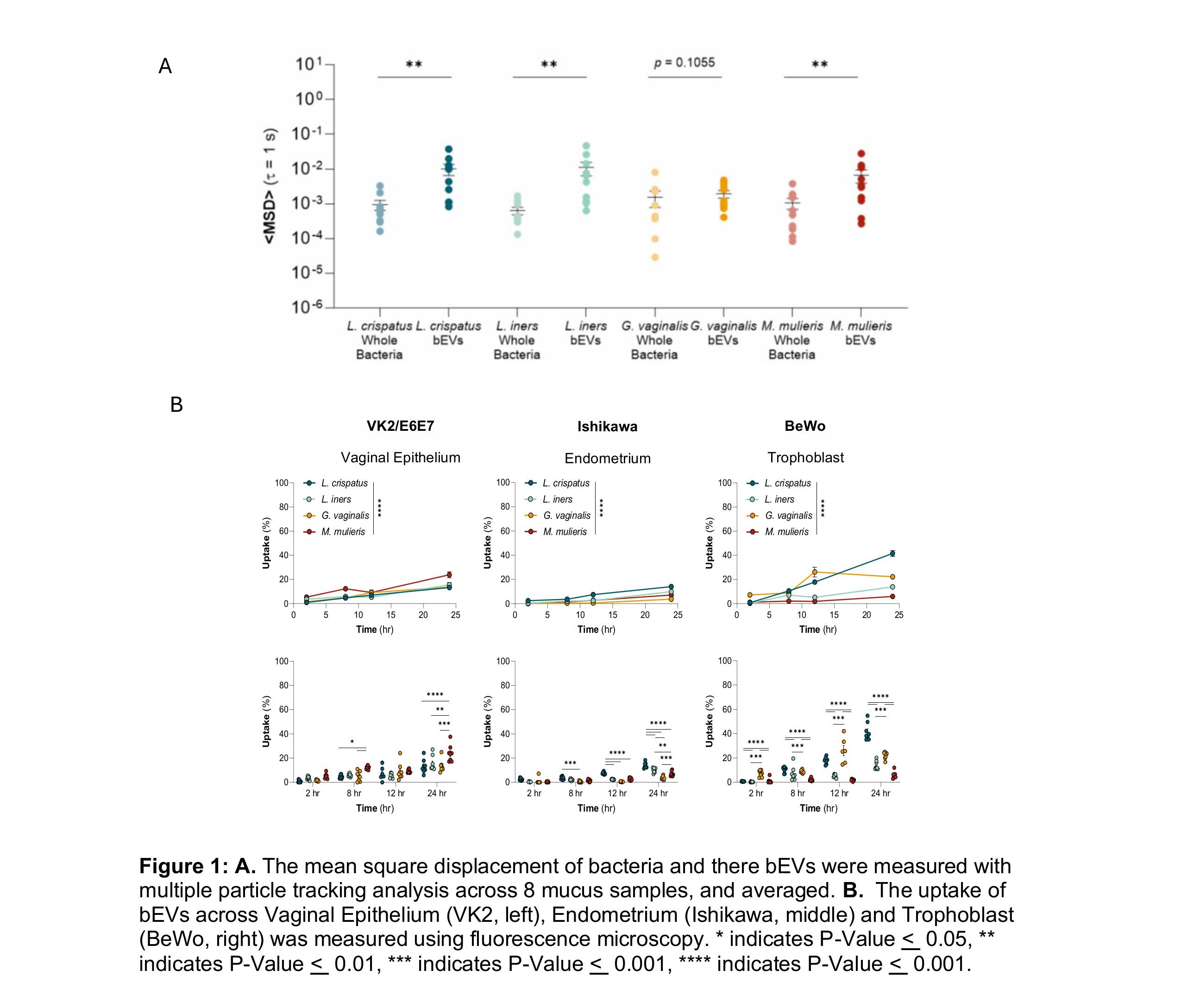
Prior work has shown cell lines derived from the female reproductive tract uptake bEVs, demonstrating their potential as a therapeutic carrier. We have shown that bEVs from different species of bacteria have distinctly different interactions with the vaginal microenvironment, despite having a similar size and surface charge (Figure 1a). We observed bEVs from L. crispatus, L. iners, and M. muleris have statistically significant higher diffusivity in mucus than their whole bacteria. Interestingly, G. vaginalis had no significant difference in diffusivity between bEVs and bacteria. Subsequently, we analyzed the difference in uptake of bEVs across different cell lines derived from the female reproductive tract (Figure 1b). We found bEVs from M. muleris have significantly higher uptake in vaginal epithelial VK2 cells across 24 hours. bEVs from L. crispatus have the highest uptake in endometrial Ishikawa cell lines. bEVs from L. crispatus also had the highest uptake in the trophoblast BeWo cell line, interestingly, bEVs from M. muleris showed the least uptake in this cell line.
Since size and surface charge of bEVs across species are similar, we believe compositional and surface chemistry differences may lead to changes in the transport properties and uptake of bEVs within the vaginal microenvironment. We hypothesize the stiffness of the bEV membrane helps control its diffusivity within the mucosal barrier. Here, we utilize atomic force microscopy approaches to measure the elastic modulus of bEV membranes and corresponding parent bacteria to determine if the elastic modulus correlates with diffusivity through mucus.6 We use scanning electron microscopy to quantify porosity of the bacterial membranes and surface morphology. Finally, we evaluate bEV uptake pathways using fluorescence staining and pathway-specific inhibitors.7 Our results have important implications for understanding species-specific mechanical properties as a readout of bEV interactions with the female reproductive tract.
This work guides two distinct avenues of research. First, the mechanobiome has been shown to be an important aspect of cell signaling and proliferation. By analyzing how bEV mechanical properties impact host cell activities, our work examines a distinct mode of microbe-host communication, which ultimately influences women’s health outcomes. Secondly, when exploring bEVs as a mode of drug delivery, modulating the mechanical properties of bEVs could impact drug delivery outcomes within the female reproductive tract. The findings here can further guide therapeutic delivery research to specific cell types within the female reproductive tract. By understanding the surface chemistry and mechanical properties of bEVs we provide insight into bEV origins, functions, and trafficking within the vaginal environment.
References
- Zierden, H. C., DeLong, K., Zulfiqar, F., Ortiz, J. O., Laney, V., Bensouda, S., Hernández, N., Hoang, T. M., Lai, S. K., Hanes, J., Burke, A. E., & Ensign, L. M. (2023). Cervicovaginal mucus barrier properties during pregnancy are impacted by the vaginal microbiome. Frontiers in cellular and infection microbiology, 13, 1015625. https://doi.org/10.3389/fcimb.2023.1015625
- Briaud, P.; Carroll, R. K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infection and Immunity 2020, 88 (12). DOI:10.1128/iai.00433-20.
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R. E. The Tremendous Biomedical Potential of Bacterial Extracellular Vesicles. Trends in Biotechnology 2022, 40 (10), 1173–1194. DOI:10.1016/j.tibtech.2022.03.005.
- Schwechheimer, C.; Kuehn, M. J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nature Reviews Microbiology 2015, 13 (10), 605–619. DOI:10.1038/nrmicro3525.
- Phillips, D. A.; Zacharoff, L. A.; Hampton, C. M.; Chong, G. W.; Malanoski, A. P.; Metskas, L. A.; Xu, S.; Bird, L. J.; Eddie, B. J.; Miklos, A. E.; Jensen, G. J.; Drummy, L. F.; El-Naggar, M. Y.; Glaven, S. M. A Bacterial Membrane Sculpting Protein with Bar Domain-like Activity. eLife 2021, 10. DOI:10.7554/elife.60049.
- Olofsson A, Nygård Skalman L, Obi I, Lundmark R, Arnqvist A. Uptake of Helicobacter pylori Vesicles Is Facilitated by Clathrin-Dependent and Clathrin-Independent Endocytic Pathways. mBio 5:10.1128/mbio.00979-14.
- Han, R.; Vollmer, W.; Perry, J. D.; Stoodley, P.; Chen, J. Simultaneous Determination of the Mechanical Properties and Turgor of a Single Bacterial Cell Using Atomic Force Microscopy. Nanoscale 2022, 14 (33), 12060–12068. DOI:10.1039/d2nr02577a.