2025 AIChE Annual Meeting
(227f) Micropillar-Induced Nuclear Deformation Promotes Bone Regeneration Via Modulation of Cell Secretome
Authors
The mPOC pre-polymer was synthesized following a previously published protocol.1 After fabrication, key properties of the flat and micropillar scaffolds were thoroughly characterized. Human mesenchymal stem cells (hMSCs) were then seeded onto the scaffolds and induced to undergo osteogenic differentiation. Secretome samples were analyzed using liquid chromatography high-resolution tandem mass spectrometry (LC-HRMS/MS) to evaluate protein expression profiles. To assess in vivo bone regeneration, a mouse cranial defect model was employed with the scaffolds. Spatial transcriptomics analysis was conducted using the Visium Spatial Platform from 10x Genomics. Statistical significance was set at p < 0.05.
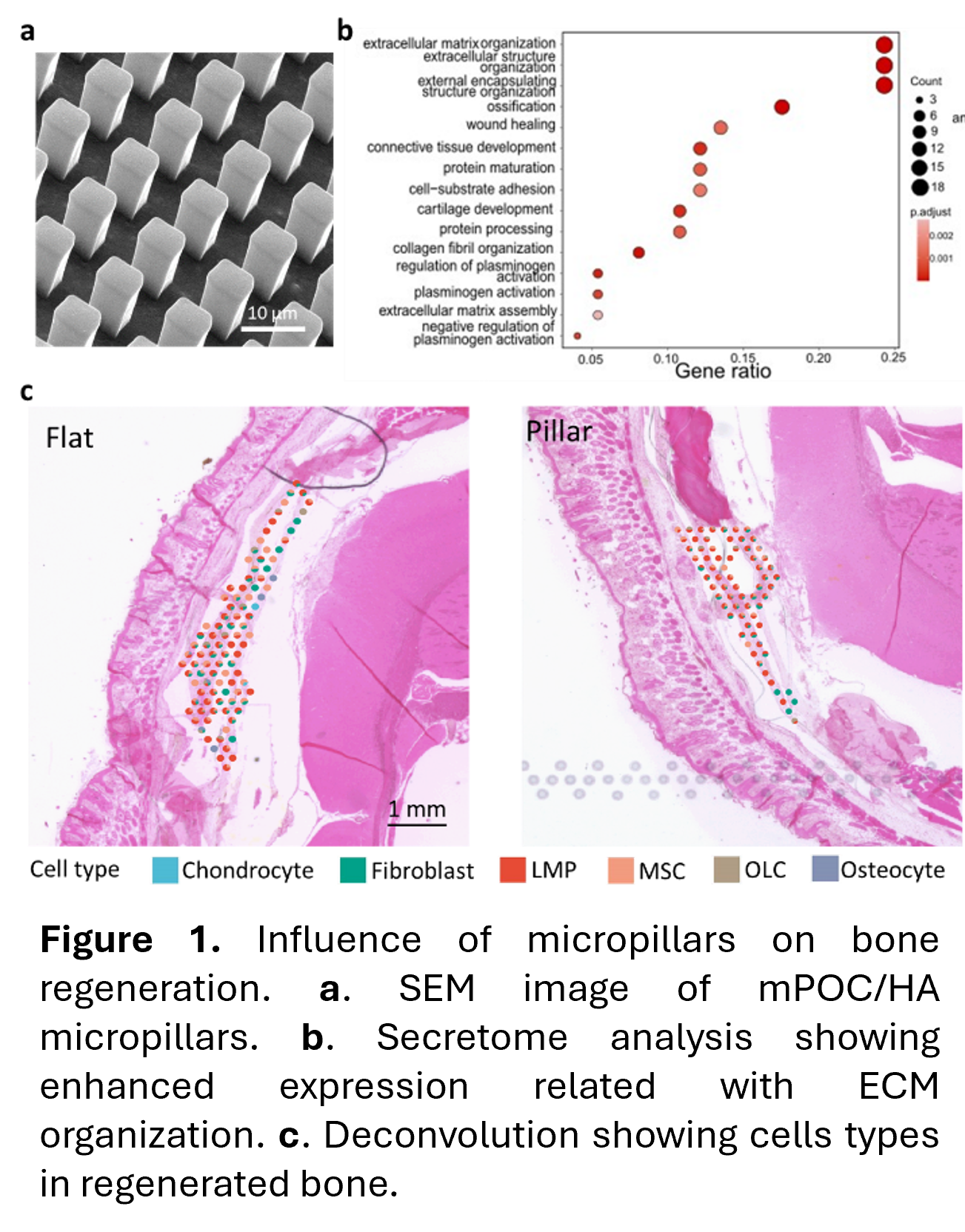
In this study, we incorporated hydroxyapatite (HA), the primary inorganic component of native bone tissue, with mPOC to create the micropillars, promoting bone formation (Fig. 1a). Our results showed that mPOC/HA micropillars facilitated osteogenic differentiation of hMSCs compared to flat mPOC/HA samples in vitro. Secretome analysis revealed that hMSCs with deformed nuclei exhibited higher expression levels of bioactive factors associated with ECM components and organization, as well as ossification (Fig. 1b). In vivo, both mPOC/HA flat and micropillar scaffolds seeded with hMSCs resulted in new bone formation; however, the micropillar group demonstrated significantly greater new bone volume and regenerated tissue thickness. Spatial transcriptomic analysis further indicated elevated expression of genes related to the regulation of ECM structures, consistent with the secretome analysis results. Additionally, tissues regenerated with micropillar implants showed an increased presence of late-stage mesenchymal progenitor cells, indicating an enhanced bone regeneration process (Fig. 1c).
In summary, nuclear-deformed cells showed increased secretion of proteins and RNA transcriptions that regulate ECM components and organization, promoting osteogenesis in neighboring cells both in vitro and in vivo. This study offers valuable insights for the future design and fabrication of bioactive implants in regenerative engineering.
References
1. Wang X, et al. Chromatin reprogramming and bone regeneration in vitro and in vivo via the microtopography-induced constriction of cell nuclei. Nature Biomedical Engineering, 2023, 7(11):1514-1529.
2. Wang X, et al. Microtopography-induced changes in cell nucleus morphology enhance bone regeneration by modulating the cellular secretome. Nature Communications, 2025, 16, 6444.