2025 AIChE Annual Meeting
(653d) Microfluidics Model Revealed Sickle Red Blood Cell?Derived Extracellular Vesicles Activate Complement System and Enhance Monocyte Adhesion on Endothelium
Extracellular vesicles (EVs) are membrane-bound nanoparticles composed of a lipid bilayer and enriched with transmembrane proteins, cytosolic proteins, RNA, and microRNA (miRNA). They serve as dynamic mediators of intercellular communication and reflect the activation state of their parent cells. Sickle cell disease (SCD) is a genetic hemoglobinopathy affecting over 300,000 newborns annually. The abnormal polymerization of deoxygenated sickle hemoglobin (HbS) leads to red blood cell (RBC) sickling, resulting in reduced deformability and promoting intravascular hemolysis, which eventually leads to chronic vascular injury. During intravascular hemolysis, SCD RBCs release RBC-derived EVs (REVs), which expose elevated levels of phosphatidylserine (PS) and carry heme. Previous publications by others and by us have demonstrated that SCD REVs (sREVs) induce endothelial cell (EC) activation and complement activation, promote abnormal cell adhesion, and trigger vaso-occlusion and organ damage in SCD. Independently, heme has been shown to suppress Kruppel-like factor 4 (KLF4), a flow-responsive transcription factor with anti-inflammatory properties. Our recent findings suggest that endothelial KLF4 may be downregulated by sREVs, further enhancing EC activation and promoting abnormal RBC adhesion. Moreover, the complement system (CS), a critical immune surveillance pathway, has been implicated in REV generation and endothelial dysfunction, yet its specific role remains understudied in human models. Given the clinical heterogeneity of SCD, we hypothesize that patient-specific sREVs contribute to complement and endothelial cell activation through the suppression of KLF4. This pathological cascade can be functionally assessed in vitro using RBC adhesion to endothelial cells as a biomarker.
Materials and Methods
To investigate SREVs activating CS and endothelial cells interaction, we have developed a novel microfluidic functional assay, the SCD-EV-BioChip, to assess REV-mediated endothelial activation by measuring cellular adhesion. ECs activated by sREVs will be used to study REV-mediated EC and complement system (CS) activation. Autologous REVs generated under shear-hypoxia and customized stress conditions will be analyzed for their pro-inflammatory effects, including KLF-4 downregulation, complement deposition, and adhesion monocyte increasing. EC and CS activation will be evaluated using adhesion assays with THP-1 cells and subject-matched sickle sRBCs on the SCD-EV-BioChip. Heme-treated and aREV-treated ECs will serve as positive controls, while ECs treated with basal media will serve as the negative control.
Results and Discussion
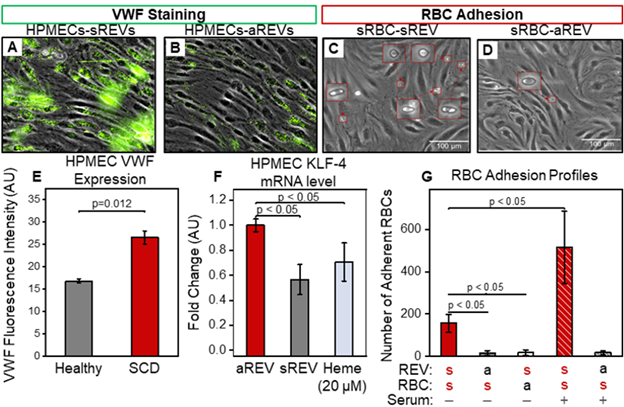
sREVs enhance von Willebrand factor (VWF) expression while reducing KLF-4 levels and promoting sRBC adhesion to human pulmonary microvascular endothelial cells (HPMECs). Treatment with REVs from SCD patients led to VWF upregulation and the formation of VWF ‘strings’ on HPMECs (Fig. 1A, B & E). sREVs significantly reduced HPMEC KLF-4 mRNA levels compared to aREV-treated controls (Fig. 1F). sRBC adhesion was observed specifically in REV-exposed HPMECs and was restricted to interactions between sRBCs and sREV-activated endothelial cells (Fig. 1C, D & G). These adhesion events were further amplified when HPMECs were incubated with REVs in normal human serum (Fig. 1G, red patterned column), suggesting a potential synergistic role of REVs and complement system activation. Collectively, these results suggest that sREVs suppress KLF-4, induce endothelial cell activation, and promote abnormal cellular adhesion, which is further exacerbated by EV-mediated CS activation.
Conclusions
Here, we present microfluidic platforms that enable in vitro assessment of sREVs and the complement system (CS) of sREVs and CS. Their impact on CS activation and endothelial function is evaluated using THP-1 adhesion as a functional biomarker under physiological flow conditions. Our experiments demonstrate that sREVs upregulate VWF expression, downregulate KLF-4, and promote monocyte adhesion to HPMECs. Notably, incubation of HPMECs with REVs in normal human serum further amplified adhesion events, suggesting REVs promotes complement activation. The microfluidic assays developed here provide clinically relevant, functional readouts that correlate with steady-state and active disease conditions, as well as treatment response, offering a potential biomarker-based platform to support diagnosis, prognosis, and therapeutic monitoring in individual patients with SCD.
Figure 1: SREV-mediated HPMEC activation. (A) HPMECs treated with sREVs demonstrated higher VWF expression (green staining) than treated with aREVs in 2 hours (B&E, p=0.012, N=5, Non-parametric Mann-Whitney). (F) sREVs and heme (as positive control) down-regulated HPMEC KLF4 mRNA levels in 4 hours compared to the ones treated with aREVs. (C, D&G) SCD-EV-BioChip RBC adhesion measurement showed RBC-endothelial interaction exists specifically between sRBCs and sREV activated HPMECs (adherent RBCs shown in red boxes). These interactions were further increased when HPMECs were incubated with REVs in normal human serum (red patterned column), indicating the potential role of REV-mediated CS activation in elevating RBC adhesion.