2025 AIChE Annual Meeting
(540f) Microbial Lysates As Low-Cost Serum Replacements in Cellular Agriculture Media Formulation
Authors
The environmental impact of meat consumption is expected to continue to rise with increasing demand and the increasing global population (OECD, Food, & Nations, 2021). The production of beef using conventional livestock agriculture is unsustainable due to greenhouse gas emissions, eutrophication, deforestation, overuse of water, and the spread of zoonotic disease (Hayek, 2022; Poore & Nemecek, 2018). Similarly, modern fishing practices have resulted in 90% of the world’s fish stocks to be overexploited or collapse (Boyd et al., 2020; Grimaldo et al., 2023). Creating meat alternatives with cell culture processes through cultivated meat technology is a potentially more sustainable alternative to these deleterious conventional livestock and fishing practices (Sinke et al. 2023). However, current cell culture practices rely on the use of fetal bovine serum (FBS), with alternatives lacking in reliability, applicability, and scalability. FBS is an expensive and animal-based ingredient with significant lot-to-lot variability and risks of contamination factors (Zheng et al., 2006; Urz et al., 2022), making its use in cultivated meat infeasible. With 90% of the cost of cultivated meat production stemming from growth media (Specht, 2020), inexpensive FBS alternatives are needed to bring cultivated meat towards commercial production. Many attempts have been made to create serum-free media (SFM) for cultivated beef and fish, but most remain expensive, labor-intensive, and unreliable. Efforts to replace FBS with recombinant albumin (Kolkmann, Van Essen, Post, & Moutsatsou, 2022; Stout et al., 2022) or other mitogenic proteins like fetuin (Skrivergaard et al., 2023) for cultivated meat production require expensive purified recombinant protein on the g/L scale. Current estimates show cultivated meat may require as much as 42 liters of media per kilogram to produce (Robert Vergeer, 2021), making the dependence on such quantities of recombinant proteins economically infeasible. Other methodologies using crude extracts or hydrolysates from plant (Stout et al., 2023) or microalgal (Dong et al., 2024; Okamoto et al., 2022) sources to supplement SFM have the potential to reduce raw material costs considerably. However, these methods can be labor-intensive, require corrosive chemicals, or are otherwise still dependent on animal serum or animal by-products. Extracts from plant-based sources, such as previously researched rapeseed isolates (Stout et al., 2023), can be difficult to produce reliably and quickly due to the relatively slow growth of plants and lot-to-lot variability inherent to seasonal crop variations.
Microbes such as yeast, bacteria, and algae provide a promising solution to the longstanding problem of creating serum-free media formulations, as they are animal-free, inexpensive to produce, highly renewable, and rich in proteins and other nutrients. Microbes can also be engineered to express growth factors, which currently represent 99% of the cost of cultured meat media at scale (Specht, 2020). However, current research focusing on microalgal and cyanobacteria extracts has not demonstrated success in identifying a non-pathogenic, rapidly replicating microbial extract capable of stimulating long-term cellular growth in serum-free conditions. Microalgae are slow-growing, taking several days to culture (Okamoto et al., 2022), while cyanobacteria can have toxic effects on cells (Chia et al., 2018). Yeast and bacterial extracts have not been shown to completely substitute for FBS or support long-term growth of animal cells (Celebi-Birand et al., 2023; Chia et al., 2018).
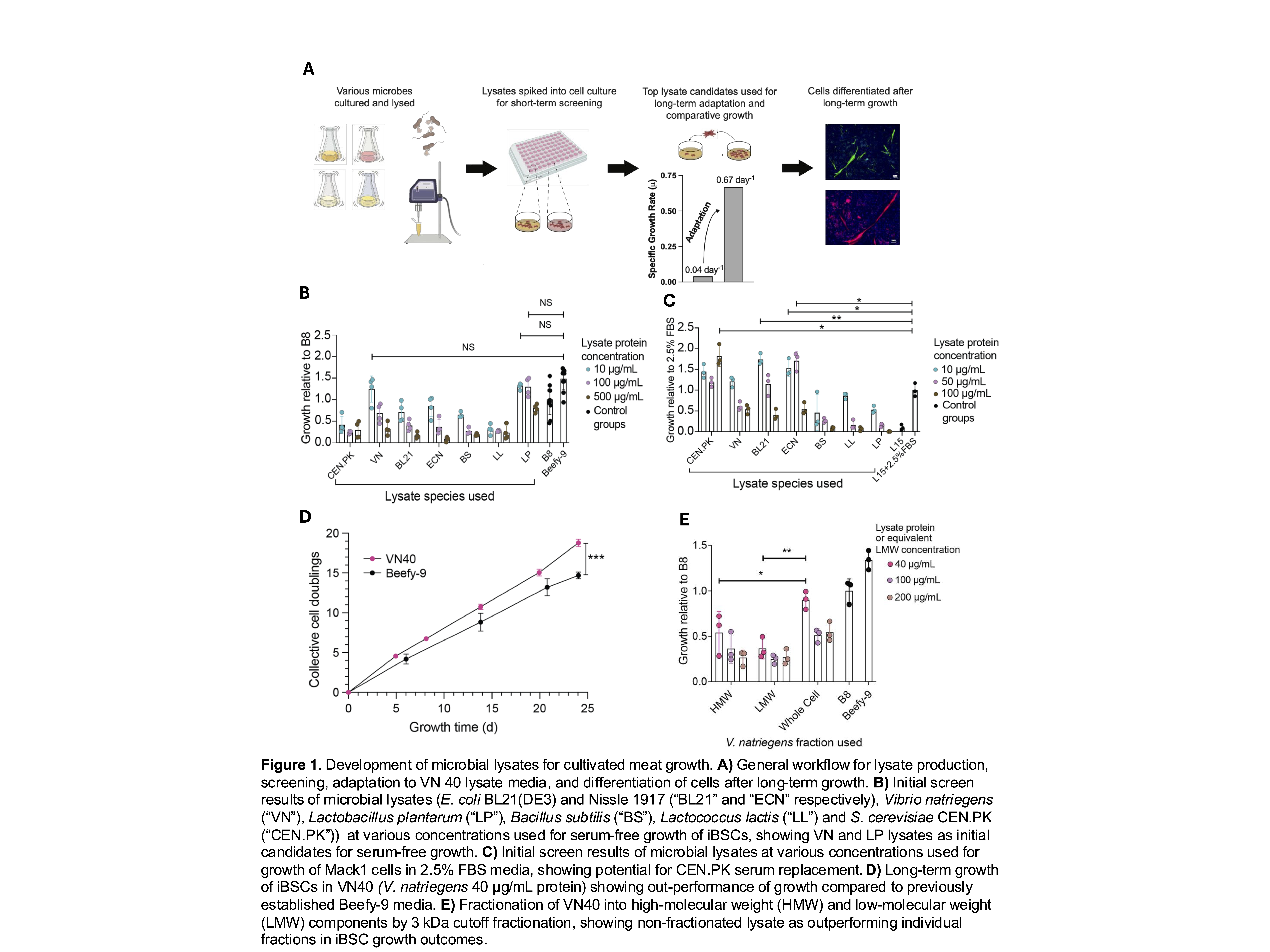
Here, we show a serum-free media produced from the food-safe and rapidly replicating V. natriegens (VN) gram-negative bacteria to be inexpensive and simple to produce, while stimulating long-term and consistent growth of bovine cells (Dolgin et al., 2025). We achieved this by testing multiple lysates from Gram-positive and Gram-negative bacteria, as well as a yeast, to determine their suitability as replacements for FBS in bovine and fish cell culture media. Based on an initial screen, we found that lysates derived from numerous microbes could serve as an alternative to FBS for immortalized bovine satellite cells (iBSCs). By further screening a smaller pool of microbial lysates using iBSCs adapted to serum-free media, we pinpointed VN lysates as a top candidate for long-term cell growth. Through adapting iBSCs to successively higher concentrations of VN lysates, we improved growth rates and arrived at an optimally performing media with 40 µg/mL VN protein, a formulation we dubbed “VN40.” Cells cultured in VN40 demonstrated robust growth, preserved their satellite cell phenotype, and the ability to differentiate into multinucleated myotubes expressing differentiation markers Desmin and Myosin Heavy Chain. At optimal growth, cells grew at rates near that of FBS-containing media, and out-grew cells grown in previously established albumin-based Beefy-9 media (Stout et al., 2022). Notably, VN lysate was able to stimulate high levels of growth at strikingly low levels of protein concentration (40 µg/mL) compared to other studies needing hundreds to thousands microgram/mL protein (Stout et al., 2022; Skrivergaard et al., 2023) for similar effect. VN is also the fastest-growing known microbe, with doubling times of <10 minutes (H. H. Lee et al., 2019) allowing for rapid conversion of cheap feedstock into a rich protein source for cell culture. Importantly, VN lysates were simple to produce, merely requiring culture of VN followed by disruption and filtration. We found that fractionation of lysates into high-molecular weight and low-molecular weight components did not aid in iBSC growth, showing that both components are necessary for VN40 performance, and that further processing of lysates is not necessary. We also conducted untargeted metabolomics of VN lysate, finding numerous nucleotides, nucleosides, aromatic amino acids, and vitamins present which are likely responsible for its mitogenic effect. We finally conducted a cost analysis of VN lysate, finding a 99% reduction in cost compared to FBS and considerable cost reductions compared to Beefy-9.
A similar screening process also identified that lysates from Saccharomyces cerevisiae could support growth of Mack1 cells (mackerel satellite cells) in reduced serum conditions. Mackerel cells have proven especially difficult to culture in serum-free conditions (Lim et al. 2024). Through this screening process, we found that with supplementation of 100 µg/mL S. cerevisiae lysate to media containing 2.5% FBS, Mack1 growth was comparable to that of media containing 7.5% FBS. This suggests the use of microbial lysates for reducing serum needs in broad cell types, and the applicability of our lysate screening process across several species.
Overall, these results highlight the promise of a whole-cell lysate derived from the fast-growing non-pathogenic marine bacterium, V. natriegens, as an easily producible, low-cost supplement for long-term serum-free growth of iBSCs for cultivated meat. There is a crucial need to replace FBS in cultivated meat applications, but current solutions are expensive, labor-intensive, and/or not suited for long-term cell culture conditions. Microbial lysates pose a potential solution, but finding viable matches for specific cell types amongst the thousands of possible food-safe microbes is difficult. Through short-term growth screens using non-adapted and serum-free adapted cells, we were able to pinpoint lysates with potential for long-term serum-free growth of iBSCs. From this, a highly potent V. natreigens lysate was discovered. Further, our approach was generalized to Mack1 cells to show its use in identifying novel microbial lysates as FBS substituents for different cultivated meat cell lines. These results point to a generalizable approach to finding cheap and easy to produce microbial lysates for serum-free and serum-reduced cultivated meat production.
REFERENCES
Boyd, R., et al. (2020). Frontiers in Marine Science, 7. doi:10.3389/fmars.2020.00639
Celebi-Birand, et al. (2023). Sustainability, 15(23). doi:ARTN 16164 10.3390/su152316164
Chia, M. A., et al. (2018). Harmful Algae, 74, 67-77. doi:10.1016/j.hal.2018.03.002
Dolgin, J., et al. (2025). Food Research International, 201, 115633. https://doi.org/10.1016/j.foodres.2024.115633
Dong, N. N., et al. (2023). Food Research International, 164. doi:ARTN 112438 10.1016/j.foodres.2022.112438
Grimaldo, E., et al. (2023). Marine Pollution Bulletin, 196. doi:ARTN 115634 10.1016/j.marpolbul.2023.115634
Hayek, M. N. (2022). Sci Adv, 8(44), eadd6681. doi:10.1126/sciadv.add6681
Kolkmann, A. M., et al. (2022). Frontiers in Bioengineering and Biotechnology, 10. doi:ARTN 895289 10.3389/fbioe.2022.895289
Lee, H. H., et al. (2019). Nature Microbiology, 4(7), 1105-1113. doi:10.1038/s41564-019-0423-8
Lim, T., et al. (2024). ACS Sustainable Chemistry & Engineering, 12(31), 11683–11691. https://doi.org/10.1021/acssuschemeng.4c03345
OECD, Food, & Nations, A. O. o. t. U. (2021). OECD-FAO Agricultural Outlook 2021-2030.
Okamoto, Y., et al. (2022). Biotechnology Progress, 38(3). doi:ARTN e3239 10.1002/btpr.3239
Poore, J., & Nemecek, T. (2018). Science, 360(6392), 987-992. doi:10.1126/science.aaq0216
Robert Vergeer, P. S., Ingrid Odegard. (2021). TEA of cultivated meat: Future projections of different scenarios -corrigendum.
Sinke, P., et al. (2023). (vol 28, pg 234, 2023). International Journal of Life Cycle Assessment, 28(9), 1225-1228. doi:10.1007/s11367-023-02183-9
Skrivergaard, S., et al. (2023). Food Research International, 172. doi:ARTN 113194 10.1016/j.foodres.2023.113194
Specht, L. (2020). Retrieved from https://gfi.org/wp-content/uploads/2021/01/clean-meat-production-volume-and-medium-cost.pdf
Stout, A. J., et al. (2022). Communications Biology, 5(1). doi:ARTN 466 10.1038/s42003-022-03423-8
Stout, A. J., et al. (2023). Biomaterials, 296. doi:ARTN 122092 10.1016/j.biomaterials.2023.122092
Urzì, O., Bagge, R. O., & Crescitelli, R. (2022). Journal of Extracellular Vesicles, 11(10). doi:ARTN 12271 10.1002/jev2.12271
Zheng, X. Y., et al. (2006). Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnology Progress, 22(5), 1294-1300. doi:10.1021/bp060121o