2025 AIChE Annual Meeting
(73f) Macrophage Regulation of Neutrophil Function in an Inflammation-on-a-Chip Microfluidic Model
Authors
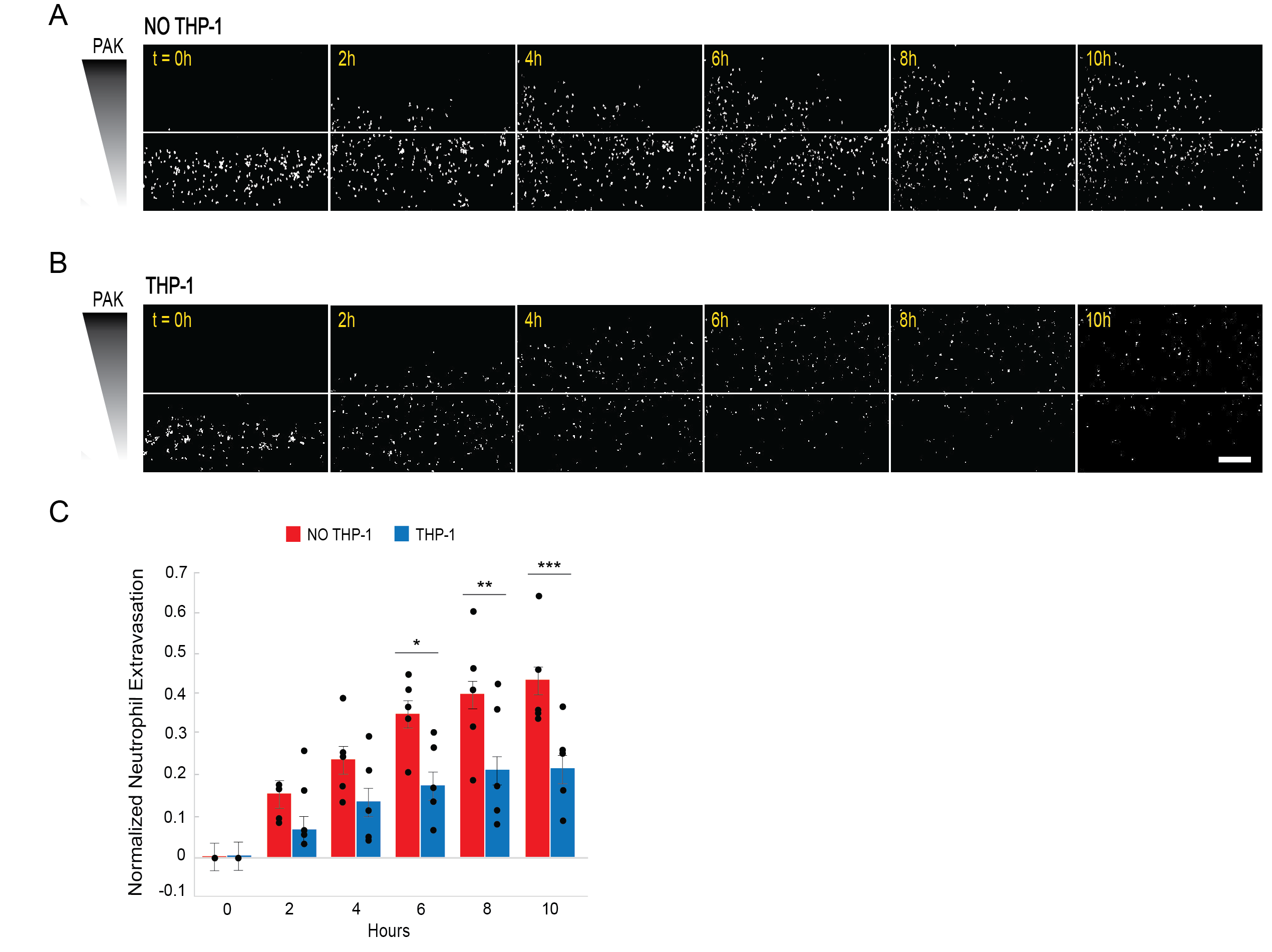
To fabricate our infection-on-a-chip model, collagen I is polymerized around a sacrificial PDMS rod. This rod is then removed, leaving behind a hollow lumen vessel. This vessel is seeded with endothelial cells to create a model blood vessel. In our initial proof-of-concept study, we embedded THP-1 macrophages in the collagen, the tissue compartment, of our infection-on-a-chip device. These cells were differentiated in the collagen over 3 days prior to endothelial cell seeding. Primary human neutrophils were then seeded in the lumens and Pseudomonas aeruginosa was added to the bacterial port to create a source of infection. Neutrophil responses were visualized using time-lapse confocal microscopy.
Our initial study found that THP-1 macrophages survived and remained differentiated in the collagen. Furthermore, we showed that they took on a M2 phenotype in this experimental platform. We found no changes to the endothelial blood vessel structure or permeability in the presence of macrophages but showed significantly reduced neutrophil extravasation when macrophages were present, indicating a change to the inflammatory microenvironment.
We are now extending these studies to instead use primary human macrophages in our device. Furthermore, we are polarizing the macrophages into M1 and M2 phenotypes to understand the differential role of pro- and anti-inflammatory macrophages in modulating neutrophil function. To date, we have found that primary human macrophages are able to readily differentiate in collagen, in our device. They remain viable and polarize to M1/M2 phenotypes under the presence of LPS/IFN (M1) or IL-4 (M2). The presence of macrophages does not alter vessel structure or permeability. We are now running extravasation experiments to understand the role of M1 and M2 primary human macrophages in altering the neutrophil response. We are running these both under infectious and sterile inflammation conditions. Furthermore, we are investigating how the presence of macrophages alters the antimicrobial functions of neutrophils including the percentage of neutrophils undergoing NETosis as this process is a critical aspect of many inflammatory pathologies.