2025 AIChE Annual Meeting
(569f) Life Cycle Assessment of Hydrogen Pipeline Infrastructure in United States: Tradeoffs between Compressed and Carrier-Based Hydrogen Delivery
Despite hydrogen's potential as a clean energy carrier, its transportation via pipelines, whether as compressed gas or chemically bound carriers (NH₃, MeOH, and LOHC), introduces greenhouse gas (GHG) emissions that must be systematically evaluated. While prior studies have extensively analyzed hydrogen delivery methods, the environmental trade-offs of transporting hydrogen via pipeline using compressed hydrogen and chemical carriers in pipelines must be explored in detail to comprehend its regional viability in the U.S.
This study aims to develop a comprehensive life cycle assessment (LCA) framework to evaluate and compare the greenhouse gas emissions associated with four hydrogen pipeline delivery methods: C-H₂, NH₃, MeOH, and LOHC. The research seeks to quantify emissions across all stages—from carrier synthesis and pipeline transport to reconversion—while examining key variables like distance, leakage rates, and energy sources. By establishing a robust methodology and identifying emission hotspots, this work aims to provide data-driven insights to guide low-carbon hydrogen transportation strategies in the U.S. energy transition.
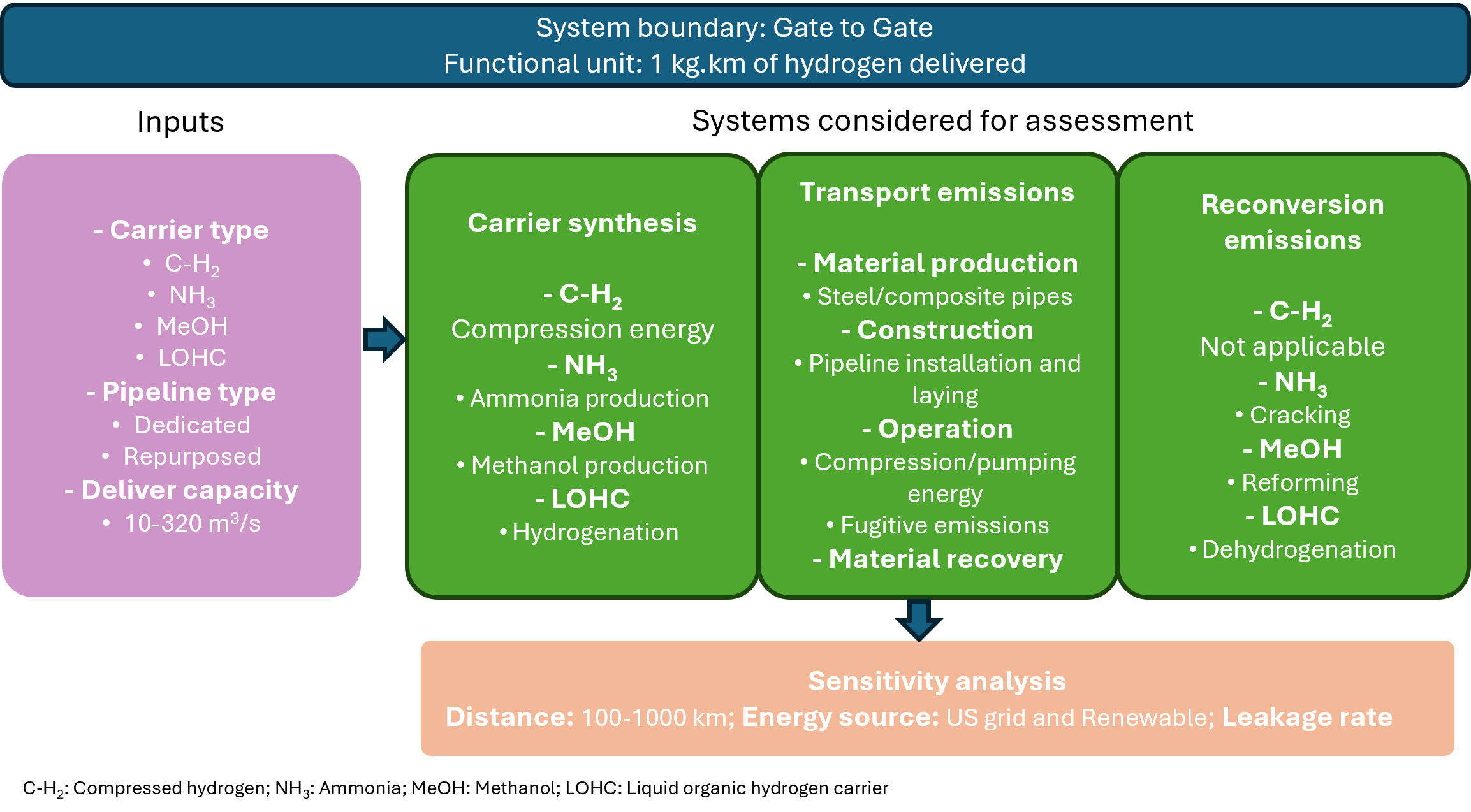
Methodology . The goal of the assessment is to evaluate and compare the potential environmental impact of hydrogen supply using pipelines. The LCA evaluates pipeline transport of compressed hydrogen (C-H₂), ammonia (NH₃), methanol (MeOH), and LOHC, focusing on the functional unit: 1 kg.km of hydrogen delivered and System boundary: Gate-to-gate emissions for pipeline transport, excluding hydrogen production and end-use.
The LCA framework integrates first-principles engineering models with spatially and temporally resolved emission factors to capture the full climate impacts of each delivery pathway. The study aims to compare greenhouse gas (GHG) emissions across different carrier configurations from perspectives on global warming potential from both 20-year (GWP20) and 100-year (GWP100) perspectives. For carrier synthesis, we account for compression energy in C-H₂ systems, Haber-Bosch process emissions for NH₃, methanol synthesis via CO₂ hydrogenation, and LOHC hydrogenation thermodynamics. Pipeline transport emissions include material production (steel or composites), construction activities, and operational energy requirements, with detailed hydraulic modeling for each carrier type - from compressible gas flow equations for C-H₂ to viscous flow calculations for liquid carriers. Given the growing concern about the potential role of hydrogen emissions in altering the climate, the latest characterization factor for the indirect global warming effect of hydrogen emissions was also included while calculating fugitive emissions. The study leverages industry databases, process modeling tools, and empirical data to estimate the comparative performance of each transport pathway.
The study incorporates sensitivity analysis to evaluate how variations in key parameters influence the overall emissions of each hydrogen delivery pathway. Monte Carlo simulations were conducted to quantify uncertainty in emission factors and operational parameters, revealing the most influential variables for each carrier system. Critical factors examined include transport distance (100–1000 km), carrier leakage rates, and energy source (U.S. grid mix vs. renewable electricity).
Expected outcomes. This study aims to provide insightful data to policymakers, stakeholders, and the public on the environmental implications of transporting hydrogen over long distances. The results would provide critical insights into the relative climate impacts of different hydrogen pipeline delivery methods, identifying key emission hotspots across carrier synthesis, transport, and reconversion phases. The analysis will quantify how factors like distance, leakage rates, and energy sources influence each pathway's total greenhouse gas footprint, helping to clarify tradeoffs between compressed gas and chemical carrier systems. The findings will guide policy decisions and industry practices by revealing the most promising transport methods under specific operational conditions. By integrating engineering models, spatially resolved emission factors, and sensitivity analysis, this work provides actionable insights for decarbonizing the energy and transport sector in the U.S. The methodology developed through this work will also establish a robust framework for ongoing evaluation as hydrogen technologies and energy systems continue to evolve.