2025 AIChE Annual Meeting
(305b) Kinetics and Temperature of Maximum Heat Release Rate for Boudouard Heat Storage and Delivery Process
Authors
Alexandre Yokochi - Presenter, Baylor University
Riley Choquette, Baylor University
Adriana Reyes, Baylor University
Tingke Fang, Baylor University
Annette von Jouanne, School of Engineering and Computer Science, Baylor University
Lulin Jiang, Baylor University
Yang Li, Baylor University
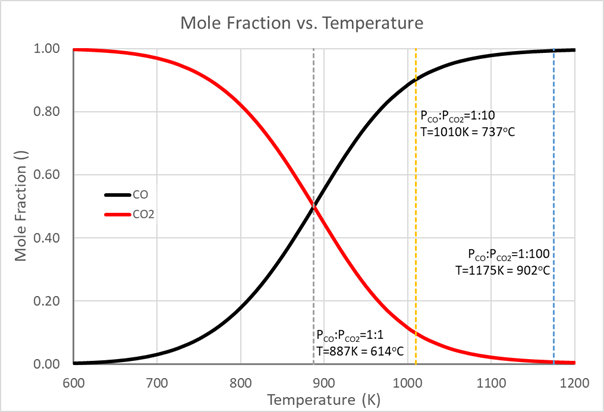
The authors have been developing the Boudouard Reaction based heat storage and delivery process, intended for long term storage of high temperature heat and it’s delivery to a distant location. The reaction, viz. 2CO ↔ C + CO2 - 171 kJ proceeds in the direction of favoring CO at high temperatures and CO2 at lower temperatures, driven by the strongly negative ΔrS term for the forward reaction resulting from the presence of more gas molecules on the left hand sidethan on the right hand side of the of the equilibrium. The equilibrium temperature at the standard pressures of PCO = PCO2 = 1bara is 614°C, but by carrying out the reaction away from standard conditions, the heat release reaction can be shifted towards significantly higher temperatures.
A key question is the rate of heat release at various temperatures and pressures. The effective rate of heat release will naturally be the difference between the rate of the forward heat releasing reaction and the rate of the reverse heat absorbing reaction, kobs = kf - kr.
In this presentation, the authors will share recent results from the measurement of the effective reaction rate, and the resulting deconvoluted rates of the forward and reverse reaction.
Figure 1. Mole fraction of CO and of CO2 with temperature of reaction.