2025 AIChE Annual Meeting
(694c) Investigation of Using Industrial Carbon Emissions to Develop Carbon-Coated Porous Silicon Materials for Energy Storage Applications
Authors
Marshall Douglas Buffett, Washinton State University (WSU)
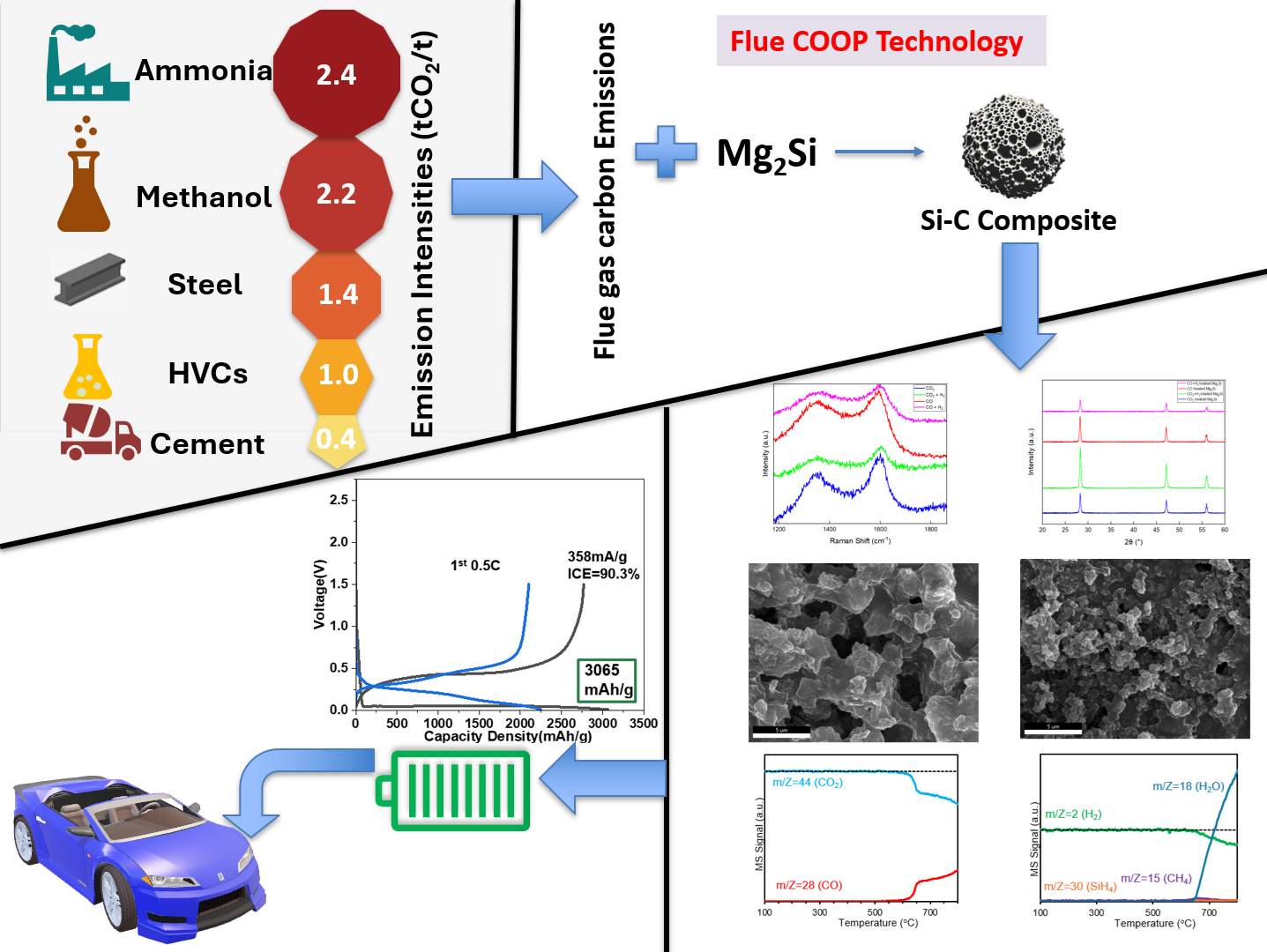
Flue gas from various industrial processes (including ammonia synthesis, steel manufacturing, petrochemical plants, cement manufacturing, methanol production, and power plants) are significant contributors to greenhouse gas emissions leading to climate change. Although steel, cement, and chemicals account for 70% of global industrial CO2 emissions, ammonia production in the chemicals sector is the largest contributor to direct emissions on a tonne-for-tonne (tCO2/t) basis, surpassing the emissions from the steel and cement industries in absolute terms. Carbon capture and utilization approaches aid in decarbonization; however, the transition to sustainable energy solutions requires upcycling of carbon emissions to develop high-value and next-generation energy storage materials. Previous research has reported the conversion of carbon dioxide (CO2) into silicon-carbon composites (Si-C), which can be used to improve the electrical performance, cycle life, and stability of lithium-ion batteries (LIBs). Our research work investigates the potential of using not only CO2 but carbon monoxide (CO) as a feedstock to develop silicon-carbon (Si-C) composites for LIBs. This approach would allow us to use more efficiently the CO produced during the reforming stage of the ammonia synthesis, which is currently converted into CO2 to eventually being vented or buried underground. In our experimental work, we used the CO2-thermic oxidation process (COOP) to convert commercial magnesium silicide into Si-C composites under four different gas environments: pure CO2, pure CO, an equimolar mixture of CO2 and H2, and an equimolar mixture of CO and H2. The XRD analysis confirmed the presence of Si with no impurities and SEM analysis showed the formation of nano to micro-porous Si structures under all gaseous environments. Raman and TGA analysis showed a higher degree of graphitization and a higher amount of carbon under a pure CO environment compared to a pure CO2 environment. The MS analysis showed that the Si-C composite formation under an equimolar mixture of CO and H2 proceeds with no side reactions, while an equimolar mixture of CO2 and H2 drives reverse water gas shift (RWGS) reaction to produce water. Our preliminary data indicates an initial coulombic efficiency (ICE) of 90.3% and a specific capacity of 3065 mAhg-1 under a pure CO2 environment. The prepared Si-C composite under a CO2 environment also showed stable cyclic performance for up to two hundred cycles. The battery performance under an equimolar mixture of CO2 and H2 showed a lower ICE (74.9%) and specific capacity (2355mAhg-1) due to the oxide formation caused by water byproduct from the RWGS reaction. The study will lay the groundwork to develop value-added materials for energy storage applications using flue gas emissions under a carbon-negative approach.