2025 AIChE Annual Meeting
(315c) Hydrothermal Synthesis of Carbon Dots Incorporated in Magnetite Iron Oxide Nanoparticles for Potential Targeted Brain Cancer Therapy: In-Vitro Study
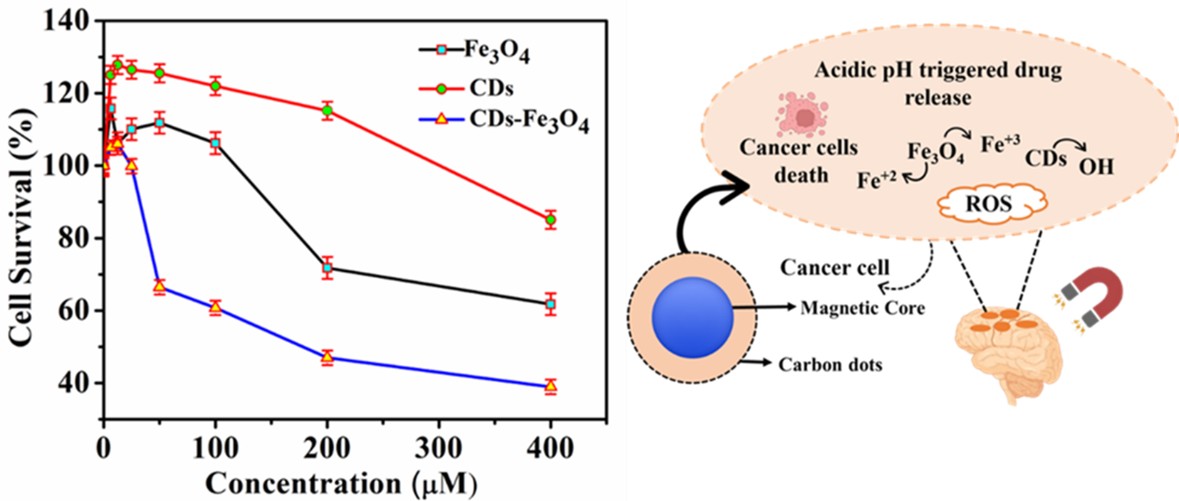
Surface modification of magnetite (Fe3O4) nanoparticles (NPs) with carbon dots (CDs) offers a promising strategy for enhancing their performance in biomedical applications, particularly due to the exceptional biocompatibility and functional versatility of CDs. In this study, CDs were successfully coated onto Fe3O4 NPs to form a composite nanostructure (CDs– Fe3O4 NPs) intended for targeted brain cancer therapy. Scanning electron microscopy (SEM) revealed that the unmodified Fe3O4 NPs possess a rough and agglomerated morphology, which was subsequently improved through surface coating. X-ray diffraction (XRD) analysis confirmed the successful incorporation of CDs onto the Fe3O4 nanoparticle surface, while Fourier-transform infrared spectroscopy (FT-IR) verified the presence of functional groups associated with both components. Magnetic characterization using a vibrating sample magnetometer (VSM) demonstrated a high saturation magnetization value of 51.362 emu/g, indicative of the enhanced magnetic response attributed to the greater occupancy of Fe2+ ions within the crystal lattice. Zeta potential measurements revealed surface charge values of -7.64 mV (Fe3O4 NPs), -0.5754 mV (CDs), and -3.881 mV (CDs– Fe3O4 NPs), confirming colloidal stability in aqueous dispersion. Brunauer–Emmett–Teller (BET) surface area analysis showed a value of 58.6 m2/g, further supporting their potential for surface-mediated interactions. In vitro anticancer assays demonstrated that the CDs-Fe3O4 NPs effectively inhibited the growth of MG-U87 glioblastoma cells. Additionally, the nanocomposite exhibited minimal cytotoxicity toward osteoblast cells, underscoring its biocompatibility and potential safety for in vivo applications. The acidic microenvironment of cancer cells enables pH-responsive drug delivery using Fe3O4 NPs, which release Fe2+ / Fe3+ ions that generate reactive oxygen species and induce cancer cell death through Fenton reactions. Notably, free Fe2+ ions produce cytotoxic hydroxyl radicals more efficiently than surface-bound Fe2+ on nanoparticles. Overall, the synthesized CDs-Fe3O4 NPs show strong promise as multifunctional agents for targeted brain cancer therapy and broader biomedical use.