2025 AIChE Annual Meeting
(183ao) Hydrophobic Counterion and Co-Encapsulated Hydrophobic Small Molecule Chemistries Influence Assembly Kinetics of Liquid Crystalline Mesophases in Nanoparticle Cores
Authors
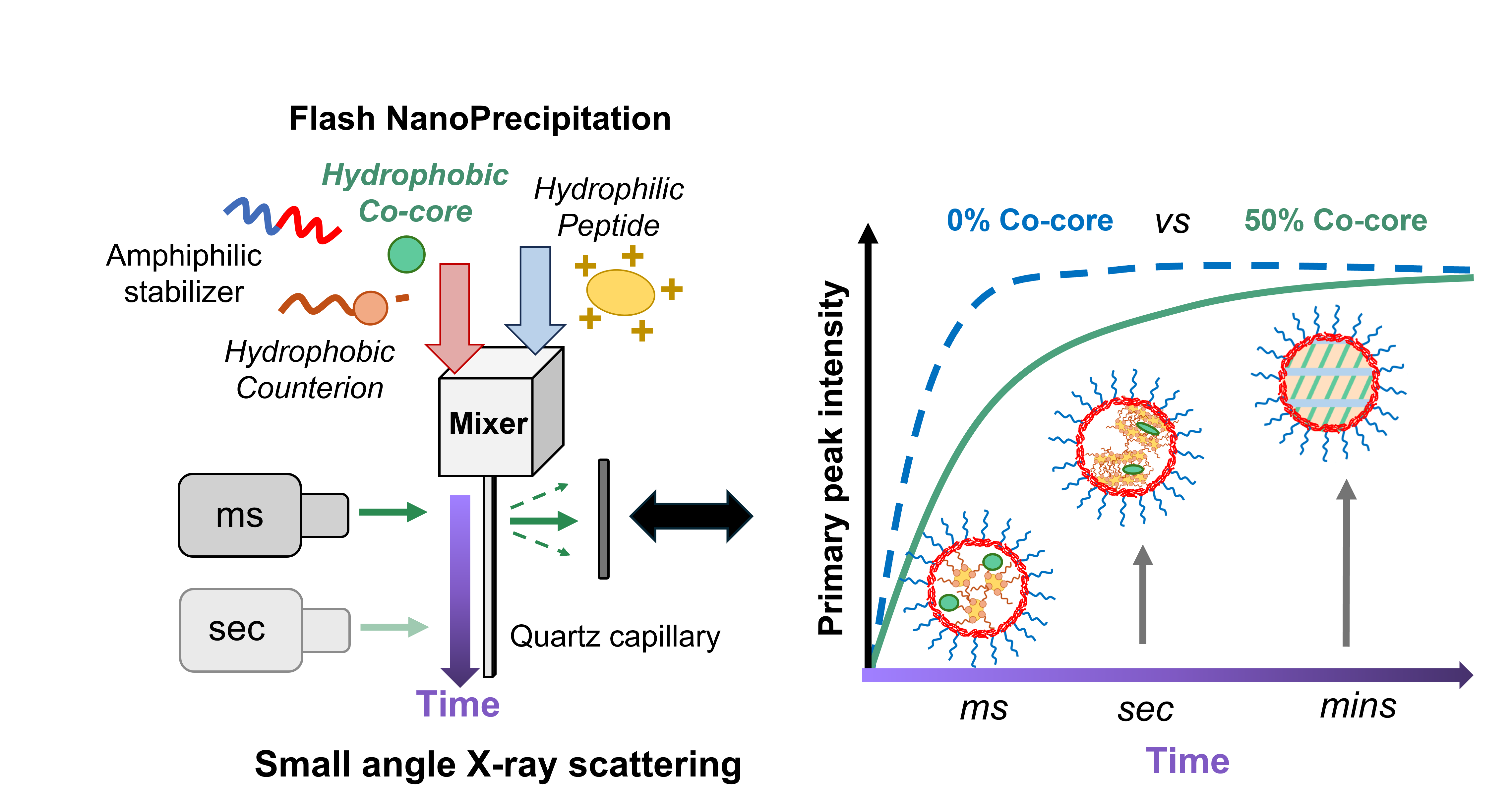
In this study, we systematically investigate the timescale over which liquid crystalline mesophases assemble in nanoparticle cores as a function of composition. Unique nanoparticle formulations comprising co-encapsulated hydrophilic (Polymyxin B) and hydrophobic (vitamin E, cholesterol, methyl oleate, or polycaprolactone) molecules—capable of generating liquid crystalline mesophases in the cores—were developed by combining Flash NanoPrecipitation with hydrophobic ion pairing using a confined impinging jets mixer. This mixer was integrated into a synchrotron small-angle X-ray scattering (SAXS) beamline to obtain spatiotemporally resolved kinetic data. During each experiment, fresh nanoparticle suspensions were produced over 60 seconds, with post-manufacture measurements taken every 90 seconds until no further structural evolution was observed.
The results of this study demonstrate that both the chemistry and concentration of the hydrophobic counterion and small molecules influence the assembly kinetics of liquid crystalline phases in nanoparticle cores. For instance, in the presence of additional hydrophobic species such as vitamin E or cholesterol, the time required for quantifiable liquid crystalline phase assembly extends to several minutes. In contrast, cores containing only polymyxin B and a hydrophobic counterion take only seconds for assembly to complete. Additionally, we find that these timescales increase with the weight fraction of the hydrophobic additive. Taken together, these findings highlight that formulation composition plays a critical role in both the assembly timeline and the characteristics of the final product—factors that are essential to control to ensure that the final liquid crystal-mediated drug delivery system maintains key quality attributes. We anticipate that these results will impact a variety of fields by providing a platform for the development of novel formulations encapsulating therapeutic cocktails to combat multi-drug resistant diseases. The presented delivery vehicle fabrication and characterization strategies uniquely integrate into continuous manufacturing processes, streamlining the scale-up of successful formulations to global platforms.