2025 AIChE Annual Meeting
(345g) Fundamentals and Engineering of Sugar Transport in Lignocellulose Degrading Thermophiles
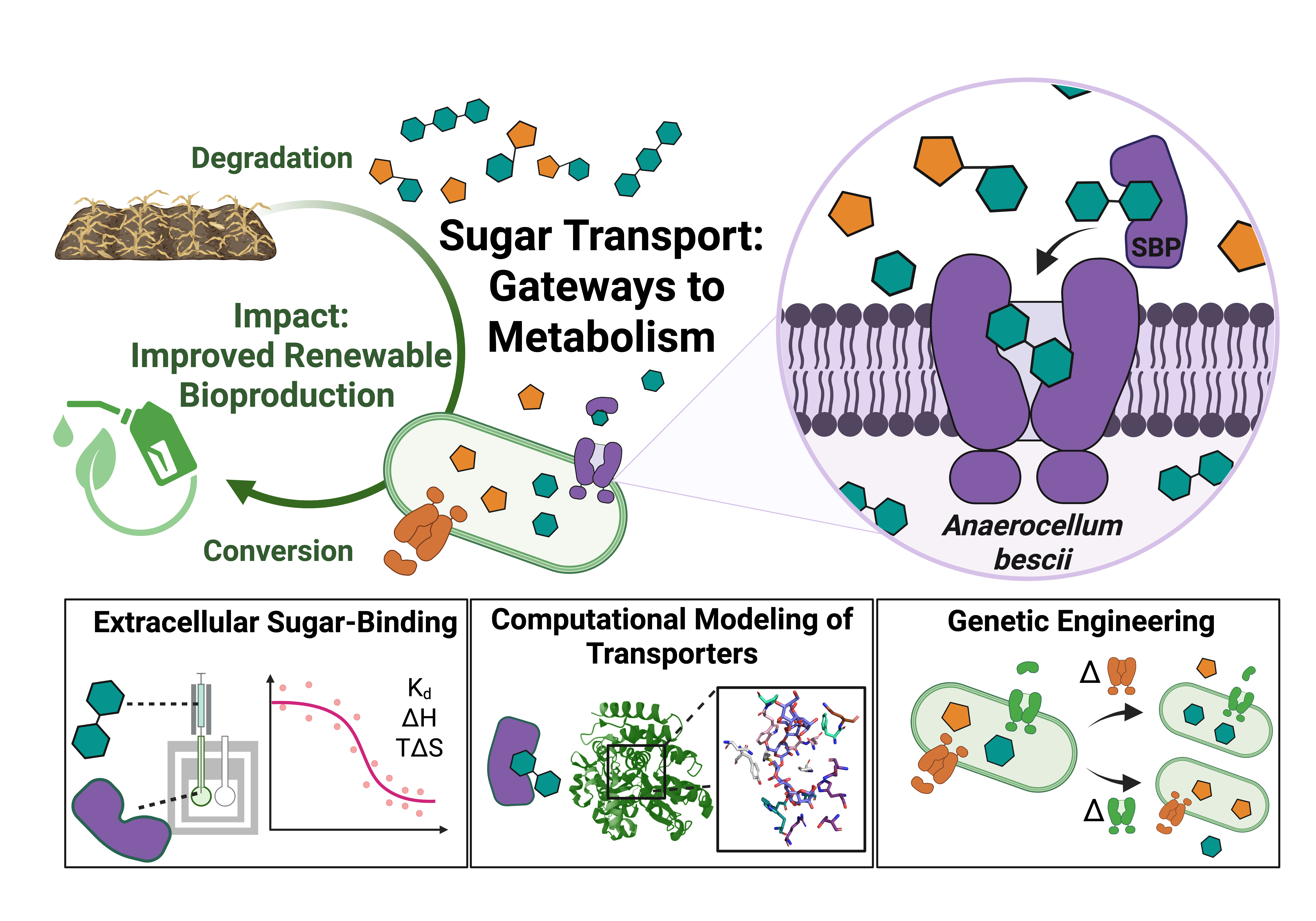
Here, we combine in vitro experiments, modeling, and genetics to investigate the substrate specificity and physiological significance of critical ABC sugar transporters in A. bescii. Using biophysical screening workflows — including differential scanning calorimetry and isothermal titration calorimetry — we have determined the substrate specificity of the sole ABC transporter for cellulose utilization in A. bescii. We genetically deleted this transporter and showed that its absence disrupts growth on cello-oligosaccharides and cellulose. We have also gleaned important structural insights into hemicellulose transport in A. bescii. We solved the crystal structure of the xyloglucan-binding protein from A. bescii, which specifically recognizes the alpha-linked xylose decorations on xyloglucan oligosaccharides. By combining fundamental structure-function insights with synthetic biology strategies to control the entry points for lignocellulosic sugars into the cell, our efforts will accelerate the development of A. bescii as an industrially important biocatalyst for producing renewable fuels and chemicals from plant biomass.