2025 AIChE Annual Meeting
(71c) Ensuring the Longevity of Medicinal Plant Cell Lines Via Cold Storage and Cryopreservation
Authors
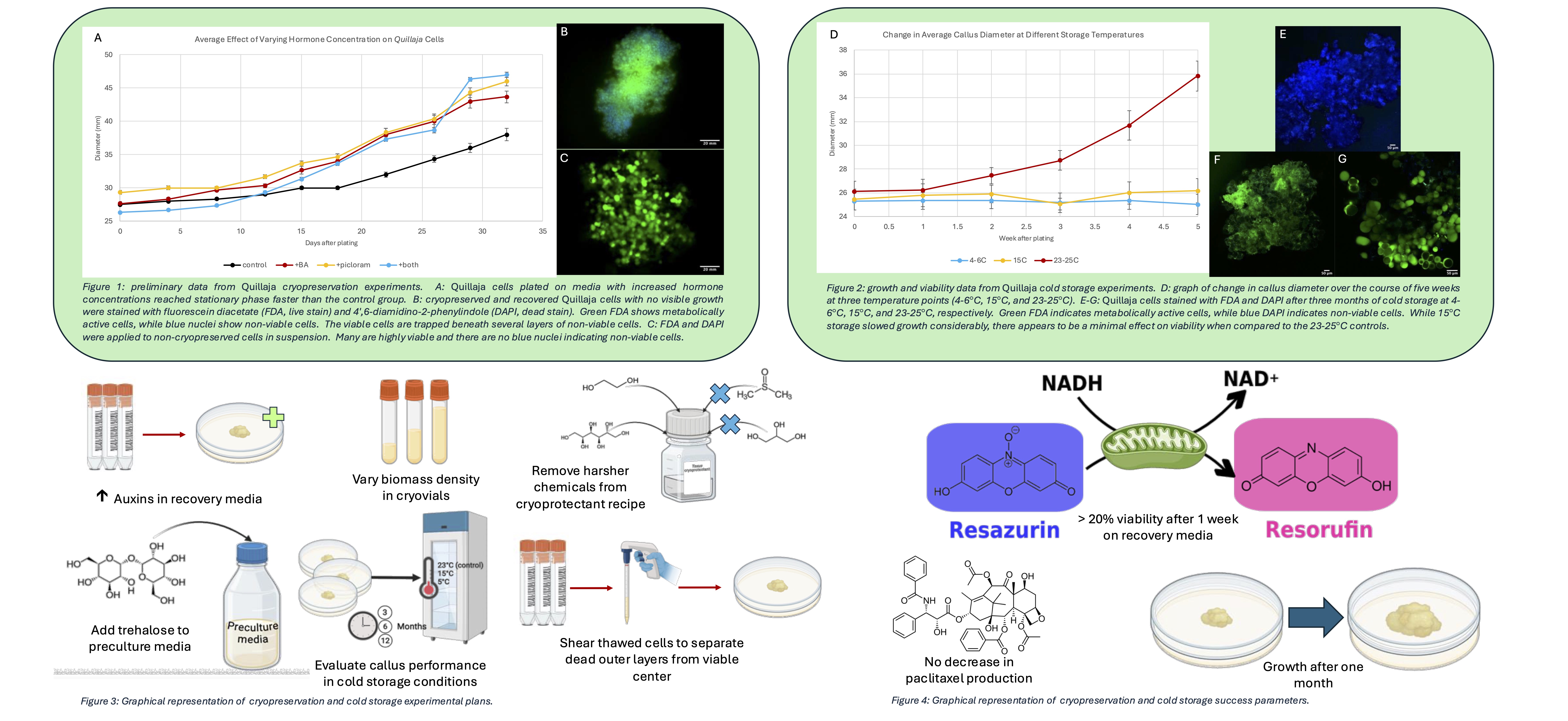
After cells are effectively cryopreserved, they must be recovered prior to initiating new cultures for biomanufacturing. Modification of hormone concentration or type can aid in both biomass scale-up and viability. When Quillaja cells were plated on media with twice the concentration of 6-benzylaminopurine (BA), they grew in diameter 24% faster than the control. When initiating new Taxus callus cultures from plant tissue, the synthetic hormone type was varied and picloram was twice as successful at forming new callus when compared to 1-napthaleneacetic acid (NAA). Four different auxin concentrations were investigated to ensure sufficient biomass accumulation during the recovery phase, and their effectiveness was evaluated by weighing the plates, measuring the callus diameter, and performing resazurin viability assays over the course of one month.
The biomass density in cryovials for frozen Quillaja cultures was not tracked in initial cryopreservation attempts. Here, three cryovials from the same origin flask were combined and plated together during the recovery phase, resulting in biomass clusters weighing between 1-1.5 g. When re-plating mature callus cultures, biomass is typically combined in clusters between 2.5-3.5 g, and when inoculating flasks, 3-5 mL of packed cell volume is added to 50mL of culture media for creation of suspension lines. Plant cells are particular about their culture conditions and react to the smallest changes (e.g., humidity differentials in the headspace of the flask). The efficacy of providing cells with consistent growing conditions by maintaining consistent cell density during the recovery phase was evaluated by weight, callus diameter, and resazurin viability assays.
In addition to preferring higher inoculation densities than yeast or bacteria (1-5 g/L and 0.3 g/L, respectively; Taxus suspensions are typically grown at 60 g/L), plant cells also do not grow as single cell suspensions because they remain attached via the middle lamella after cell division. Since aggregates range in size from two cells to hundreds, cells in each aggregate experience different microenvironments. Cells at the center of an aggregate are protected from environmental disturbances, but they also receive less nutrients and signaling compounds from the surrounding culture media when compared to the outermost layer of cells. Microscopy images showed that cells at the center of an aggregate were viable after recovery, but they were surrounded by several layers of non-viable cells. This result suggests a potential strategy where disaggregation of recovered cells via shearing may result in faster recovery as dead outer layers will be removed and enable viable cells direct access to nutrients in the media.
While experiments in Quillaja focused on issues during the active cryopreservation and recovery process, improvements can also be implemented prior to cryopreservation. Sugar alcohols are often used in media as a replacement for sucrose before cryopreservation to dehydrate cells and protect them from lysing due to ice crystal formation. Quillaja protocols were developed from previous studies in Taxus and other systems that demonstrated sorbitol and mannitol to be superior osmotic agents in the preculture medium when compared to trehalose and proline. Initial attempts at Quillaja cryopreservation involved preculture media made with either 0.5M sorbitol or 0.5M mannitol; however, both formulations demonstrated zero biomass accumulation as determined by weight and callus diameter after recovery. A wider range of osmotic agent concentrations and types (0.1-0.8M sorbitol, mannitol, and trehalose) were investigated for increasing recovered callus growth by weight and diameter, as well as viability by resazurin assay.
We will also report on data using cold storage as an alternate preservation method. In experiments with Quillaja cells that are derived from tree samples that grow in a warmer and drier climate than Taxus, it was found that storage of callus plates at 15°C had little negative effect on viability but slowed primary metabolism by a factor of 3.4. Temperatures as low as 4-6°C were also tested, but after six months, cells were no longer viable. Taxus plates were stored at 15°C and 4-6°C and examined monthly for changes in viability, as well as sampled every three months for epigenetic changes and paclitaxel production. Cold storage may be an effective alternative to cryopreservation, as it is less costly, less energy-intensive, and can result in more rapid scale-up of cultures. The insights we have gleaned from the Quillaja and Taxus systems can be used in the development of protocols for the preservation of new plant systems initiated for biomanufacturing, enabling more widespread industrial use of plant cell culture.