2025 AIChE Annual Meeting
(640e) Enhancing Electrocatalytic Conversion of CO2 Via Bicarbonate Reduction to Formate
Author
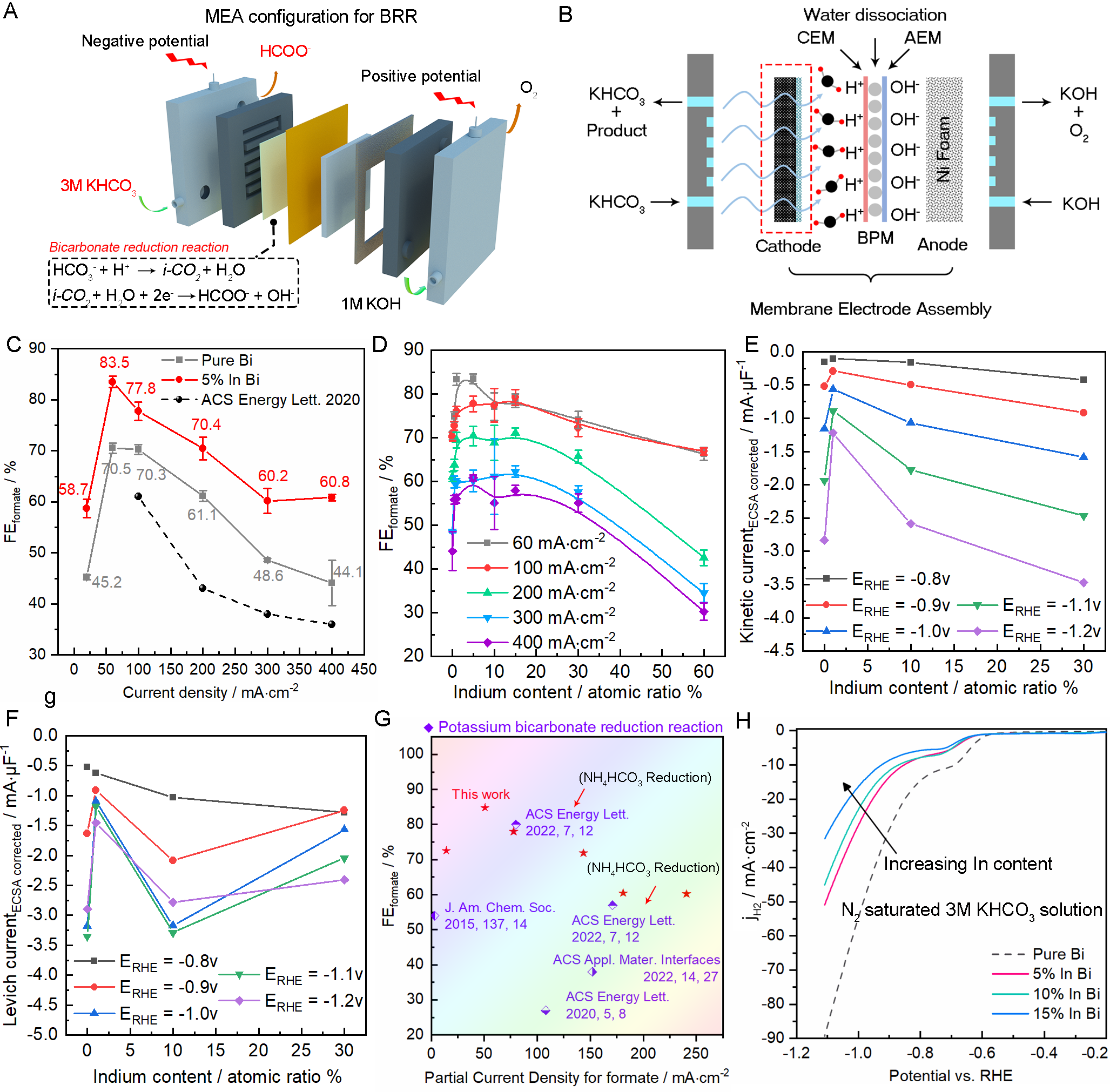
Herein, we introduced innovations to the catalyst microenvironment, catalyst composition, and reactor configuration to improve selectivity. In terms of catalyst composition, we introduced a low hydrogen-affinity metal (indium) into bismuth-based materials for BRR in order to improve selectivity over the competitive HER. It shows a favorable FEformate of 84.81%, a considerable formate partial current density of 241 mA∙cm-2, and a superior formate generation rate of 56 mmol∙L-1∙cm-2∙h-1 compared to current literature reports.
Our comprehensive ex-situ XRD characterization suggests that the incorporation of indium inhibits the phase transformation from metallic bismuth to a bismuth subcarbonate species, which leads to the enhanced performance for bicarbonate reduction to facilitate improved cathodic formate production from the In-Bi alloy as compared to pure Bi.
This presentation will provide further perspectives into how the microenvironment for bicarbonate reduction should be controlled to improve selectivity.