2025 AIChE Annual Meeting
(227b) Enhancement of Mechanical and Morphological Properties of Agarose Hydrogels Via the Incorporation of Isocyanates for Cartilage Tissue Engineering
Authors
In this preliminary study, two distinct isocyanates (Desmodur N 3300A and hexamethylene diisocyanate) were incorporated in agarose gel systems to study their impact on key chemical, mechanical, and morphological properties. Desmodur N3300A (Desmodur) is a cyclic polyisocyanate that features NH and CO groups that promote extensive hydrogen bonding, while hexamethylene (HDI) is a linear molecule with CH chain backbone. The resulting hydrogels were characterized using FTIR to confirm chemical bonding, rheology to assess dynamic mechanical properties, SEM to confirm morphology, and swelling analysis to compare water uptake.
Methods and Materials. In this study, agarose hydrogels of 1, 3, and 5 wt% were modified by incorporating Desmodur (D-Agar) and HDI (H-Agar). Isocyanate-modified agarose was achieved by adding 5.2 x 10-4 NCO molar equivalents (i.e., corresponding to each isocyanate amount) and catalyst (Coscat 83) to 1 gram of agarose powder. The mixture was blended in a speed mixer at 2500 RPM for 2 minutes and 30 seconds to ensure homogeneous distribution of the diisocyanate and catalyst. Agarose control hydrogels were used as provided. Agarose hydrogels were prepared by weighing the appropriate amounts of agar powder (either control or modified) to the target concentrations in 1X Tris buffer (pH 7.4). The mixtures were heated to 120 °C for 10 minutes or until the solution was clear, indicating complete dissolution. The hot agarose solution was poured into individual wells of the well plate and allowed to cool undisturbed at room temperature for 5 minutes until gelation.
Samples were analyzed using FTIR to determine the degree of potential urethane or urea bond formation in samples containing isocyanate molecules. SEM micrographs were obtained to assess pore morphology and qualitative size distribution. Dynamic mechanical properties were assessed using a rheometer in oscillatory mode at 25 °C. Swelling studies were conducted by immersing dry hydrogels in buffer over a 24-hour period. All data was analyzed across different agarose concentrations and isocyanate types.
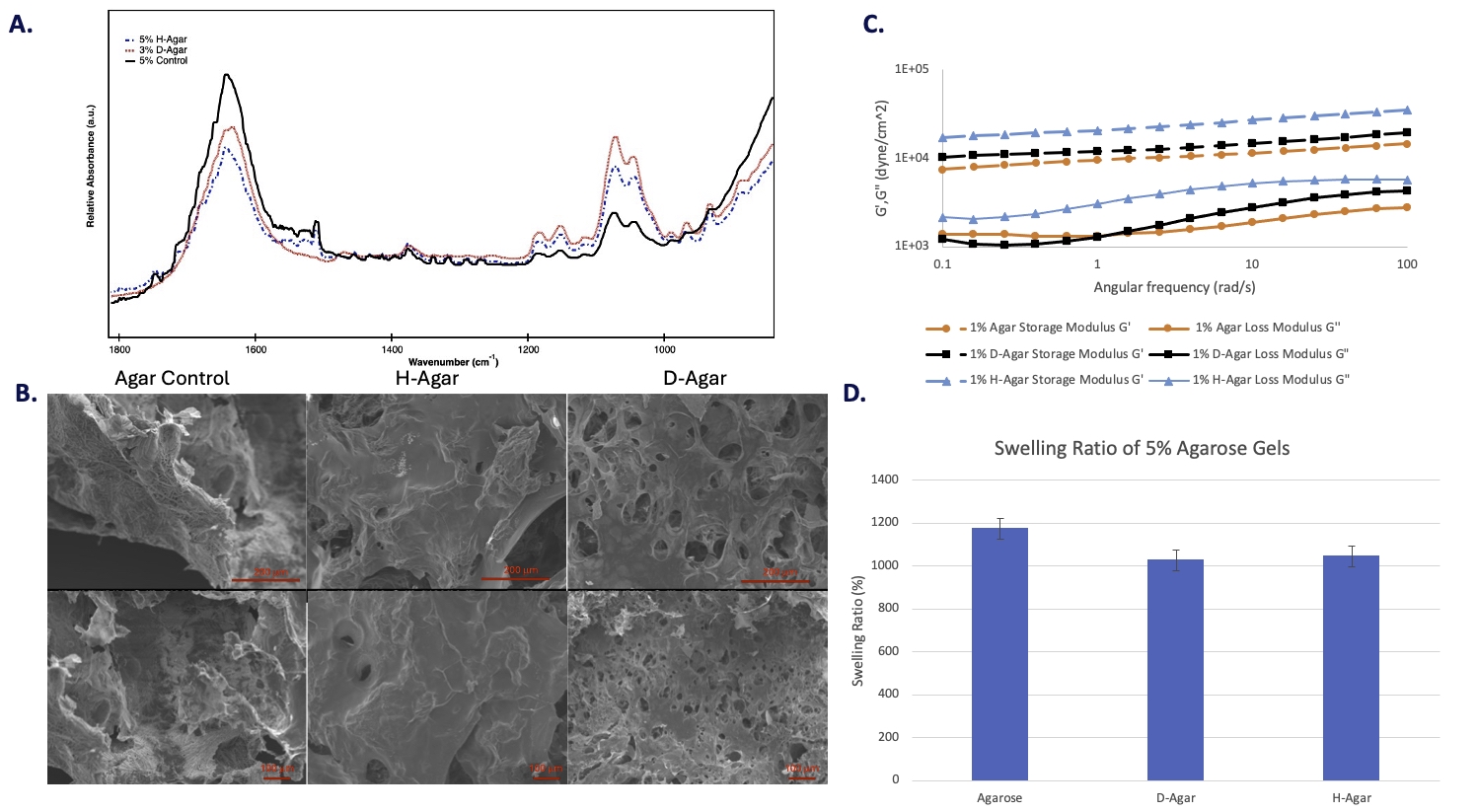
Discussion. FTIR analysis (Figure 1A) of wet hydrogel samples indicated a total consumption of NCO groups from isocyanates as the 2260 cm-1 peak was absent in all spectra. Urethane nor urea bonds were observed in the FTIR spectra. An increase in absorbance and slight red shift in -CH and -C-O bending signals in the fingerprint region (800 cm-1 – 1200 cm-1) implies there is increased strain on the glycosidic bond and C-O-C bridge of the 3, 6-anhydro-a-L-galactose unit of agarose and subsequent hydrogen bonding following isocyanate incorporation. Decreases in -OH bending near 1640 cm-1 and -OH stretching near 3400 cm-1 in the spectra suggest reduced water content within the hydrogel as a result of enhanced crosslinking and hydrogen bonding interactions. Desmodur was observed to have the greatest influence on peak shifting in the fingerprint region and OH peak intensity.
SEM micrographs (Figure 1B) of lyophilized 5 wt% hydrogels revealed the morphological changes as a result of incorporating isocyanates in the hydrogel system. The modified agarose gels exhibited an increase in pore size, transitioning from undefined, nanoscale pores in the unmodified agarose gels to defined pores with diameters ranging from approximately 5.5 to 170 micrometers in the modified agarose. The results from the SEM suggest that the incorporation of isocyanates increases the porosity of agarose hydrogels. As expected, this increased porosity may be attributed to the production of carbon dioxide gas resulting from the blowing reaction of the NCO groups with water. The pore formation is significantly increased for D-Agar gels as compared to H-Agar gels. This observation is likely as a result of Desmodur’s third isocyanate group, which promotes increased reactivity.
Preliminary rheology data (Figure 1C) indicated that the incorporation of isocyanates improved the storage modulus (G’) of the hydrogels when compared with agarose control samples. This observation was expected as the incorporation of isocyanates promotes chemical interactions (i.e., greater crosslinking). However, the storage modulus of H-Agar hydrogels was greater than D-Agar hydrogels. This result magnifies the counterplay between pore formation and the degree of crosslinking with respect to dynamic mechanical properties. The incorporation of isocyanates also increased the loss modulus (G”) of agarose hydrogels, which indicates that the incorporation of isocyanates does not negatively impact the flowability of the materials.
The results from the swelling study (Figure 1D) of lyophilized hydrogels support the FTIR data, as it was demonstrated that hydrogels made from isocyanate-modified agarose had a reduced capacity for swelling compared to the pure agarose. The incorporation of Desmodur reduced hydrogel swelling by 150%, while HDI incorporation reduced swelling by 130%. These differences in the impact on swelling between HDI and Desmodur agarose are most likely the result of increased reactivity and hydrogen bonding from Desmodur, promoting a greater crosslink density than HDI.
Summary. Preliminary results from this study have shown that the incorporation of isocyanates in agarose hydrogels produces a more porous system with reduced swelling and improved dynamic mechanical properties. The reduced swelling and improved dynamic mechanical properties suggest that isocyanates promote additional crosslinking in the hydrogel (e.g., via hydrogen bonding). As Desmodur is a more reactive as a cyclic isocyanate, its incorporation in the agarose hydrogel produces more pores in the system than the incorporation of HDI. Further, FTIR results suggest that isocyanates impact crosslinking in the system as changes in CH, CO, and OH peaks were observed. Both improvements in dynamic mechanical properties and pore formation are critical features in cartilage tissue engineering, overcoming the current limitations of classical agarose hydrogels. Ongoing work includes dynamic mechanical analysis using temperature sweeps, thermal characterization, release studies of biomolecules, and studying the impact of isocyanate percentage to tune hydrogel porosity.
References.
- 1. Jeyaraman, M., et al., Critical challenges and frontiers in cartilage tissue engineering. Cureus, 2024. 16(1).
- 2. Karunanithi, S. and K. Rajappan, Biofunctionalized agarose-based biopolymer composites for advanced biomedical applications: a review. Polymer Bulletin, 2025: p. 1-43.
- 3. Salati, M.A., et al., Agarose-based biomaterials: opportunities and challenges in cartilage tissue engineering. Polymers, 2020. 12(5): p. 1150.
- 4. Sonker, A.K., et al., Crosslinking of agar by diisocyanates. Carbohydrate polymers, 2018. 202: p. 454-460.