2025 AIChE Annual Meeting
(640c) Engineering the Temperature of CO2 Electrolyzers for Selective Multi-Carbon Chemicals Manufacturing
Authors
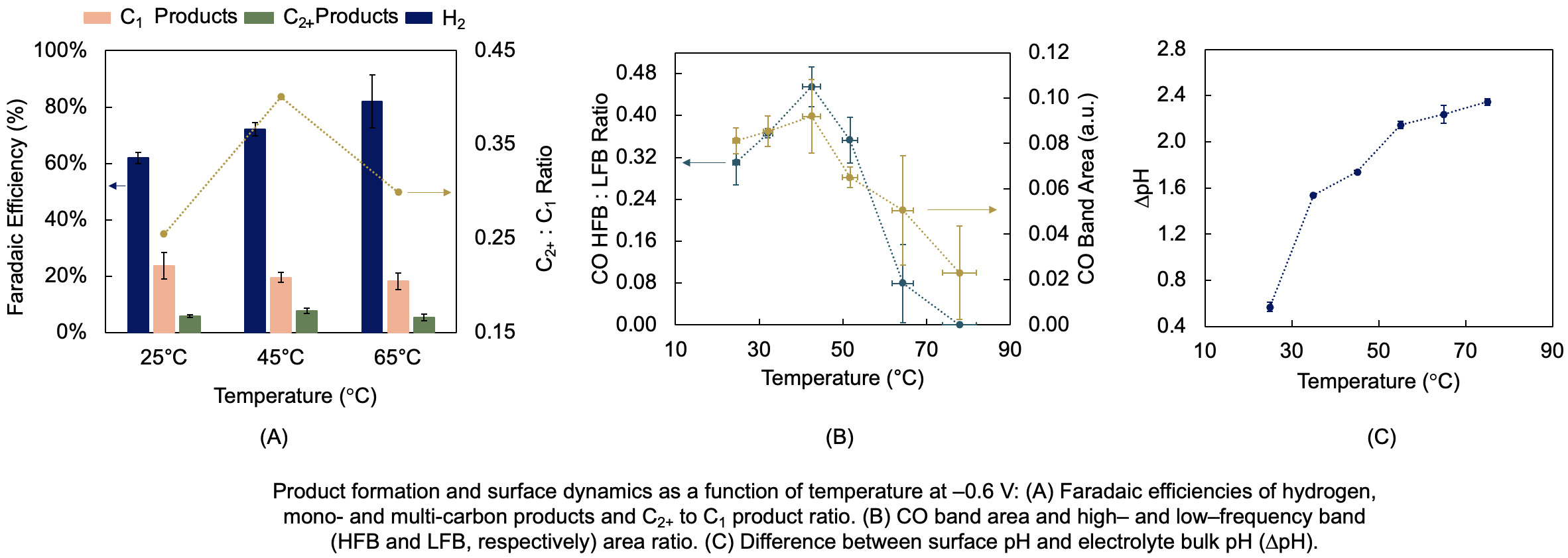
A maximum in multi-carbon products selectivity was observed at 45°C (Fig. A). With SEIRAS, we showed that there are two temperature regimes controlling the surface population of the reactive CO intermediate: one between 20 and 45 °C, where CO coverage and migration rate to defect sites increase; and one between 45 and 80 °C, where CO coverage and coupling rates decrease (Fig. B). With SERS, we revealed that higher temperature leads to stronger pH gradients consisting of greater surface to bulk pH differences over thinner boundary layers (Fig. C). Together, our results suggest that high CO coverage and moderately alkaline surface pH is beneficial for multi-carbon products formation below 45 °C. Above 55 °C, however, low CO coverage and too high surface pH favor hydrogen evolution and compromise carbon–carbon coupling. Using surface sensitive techniques to better understand the effect of temperature on the reaction is a key step to design optimal solutions targeting multi-carbon products manufacturing in practical CO2 electrolyzers.