2025 AIChE Annual Meeting
(288a) Design and Evaluation of ApoE-Functionalized Polymersomes for CNS-Directed Enzyme Delivery in GM1 Gangliosidosis
Authors
Methods: Synthesis of polymersomes was performed using PEG(1000)-b-PLA(5000) polymer dissolved in dimethyl sulfoxide. Solvent injection of this solution into an aqueous phase created polymersomes that were lyophilized to increase stability. Similarly, ApoE functionalization of polymersomes occurred during synthesis and involved the addition of an N-hydroxysuccinimide linker in the organic phase and full length ApoE protein in the aqueous phase. Polymersome formation was confirmed using dynamic light scattering (DLS) and Zeta Potential. Application of the Lowry assay was used to determine the effective functionalization of polymersomes with ApoE. Polymersomes were loaded with a hydrophilic Cyanine 5 (Cy5) or a fluorescently tagged β-gal using a dialysis method in combination with UV visibility plate reader to confirm encapsulation percentage and loaded content by mass. Knockout mice heterozygous for the production of β-gal were provided by Jackson Laboratories and bred to produce mutant mice with extremely limited production of β-gal enzyme. Mouse genotyping was performed using polymerase chain reaction (PCR) To determine the effectiveness of ApoE functionalized polymersomes in crossing the BBB, mice were injected with ApoE or unfunctionalized polymersomes containing Cy5 at a dose of 2400 mg polymersome/kg of bodyweight and in vivo imaging system (IVIS) and blood draws were taken at 0.25, 1, 4, and 24 hours. At the 24-hour time point, mice were sacrificed, and organs were harvested for further processing. IVIS images of organs including the brain, spinal cord, liver, heart, spleen, and kidneys were performed on the same scale to demonstrate differences in fluorescence within the organs. Organs were fixed and sectioned onto slides for immunofluorescent experimentation wherein antibodies for neurons, Cy5, and 4',6-diamidino-2-phenylindole (DAPI) were used to identify areas of colocalization between Cy5 and neurons. This was further confirmed using confocal microscopy Enzyme delivery efficiency was determined by using a similar setup as the previous approach, however, for a total of 48 hours. Mice were injected with ApoE functionalized polymersomes at the same dose as the previous experiment and blood was drawn at the same time points but with an additional time point at 48 hours. At the final time point, mice were sacrificed, and organs were collected and stored in either formalin or Optimal Cutting Temperature (OCT). Enzyme delivery was demonstrated using enzyme assays on sectioned tissue as well as blood serum for β-gal, Hexosaminidase A and T, and Mannosidase taking advantage of the 4-methyl umbelliferyl substrate family. Brains were sectioned into a frontal, mid, and rear sections for analysis. Furthermore, qualitative distribution to cells was demonstrated using the colorimetric 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining on slides prepared from OCT stored tissues.
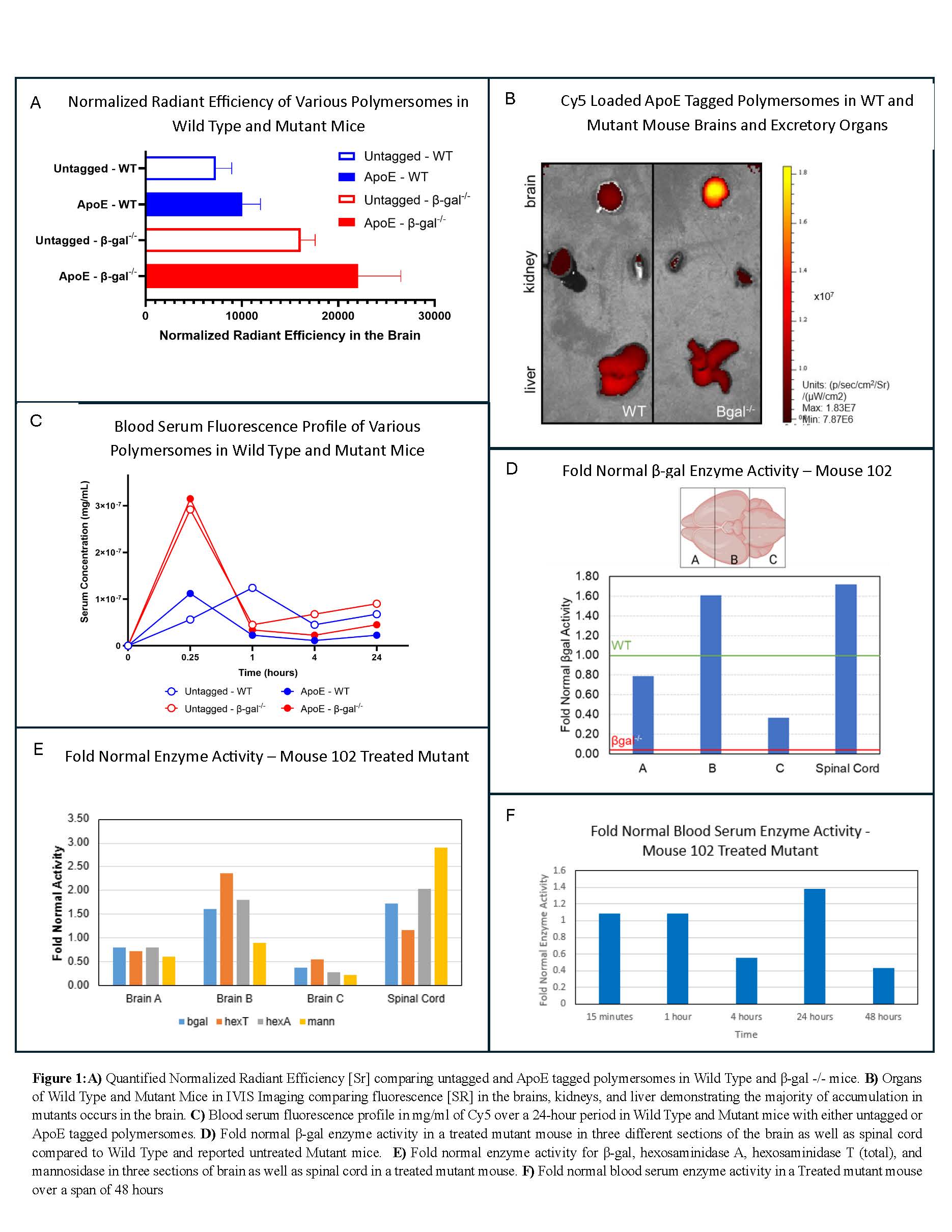
Results: Unfunctionalized polymersomes had a mean diameter of 147±17nm with a Zeta Potential of -19.4±3.4 mV. ApoE functionalization resulted in an increase in diameter to 169±18nm and a slight decrease in Zeta Potential to -15.6±9.1 nm due to the addition of ApoE and its variable charge. Lowry results further confirmed an increase in protein from 0.01 mg/ml in untagged polymersome samples to 0.67 mg/ml in ApoE functionalized samples. After dialysis, unfunctionalized polymersomes had a loaded Cy5 content of 0.017±6.8E-4 µg/mg of polymersome and ApoE polymersomes had a content of 0.013±5.2E-4 µg/mg of polymersome. Β-gal loading of all polymersomes resulted in a loaded content of 0.01±0.001 mg/mg polymersome as determined by fluorescent spectroscopy. Biodistribution results demonstrated 1.5 times increased fluorescence in the brains of mutant mice when using ApoE polymersomes as compared to mice with unfunctionalized polymersomes administered and 3 times greater than wild type mice with any polymersome administered (Figure 1A). Brains of mice were nearly twice as fluorescent as livers indicating a lack of accumulation in clearance organs (Figure 1B). Analysis of blood serum fluorescence indicated a sharp spike in fluorescence at 0.25 hours followed by a decline over time in fluorescence (Figure 1C). Crucially, mice administered with ApoE polymersomes reached lower serum fluorescence values and maintained them for longer times which is hypothesized to be due to ApoE’s internalization into tissues. Immunofluorescence experiments indicated that Cy5 reached neurons as shown by colocalization of Cy5 antibodies with neuronal antibodies. Therapeutic treatments demonstrated β-gal activities reaching fold normal values of 1.6 in brain section B and the spinal cord, 0.8-fold normal in brain section A, and 0.38 in brain section C (Figure 1D). Untreated mice are reported to have a fold normal enzyme activity of only 0.03 demonstrating a massive increase in β-gal activity in neural tissue and successful delivery of enzyme. Additionally, secondary markers like the hexosaminidase family and mannosidase enzymes are decreased to between 1.5 and 2.5 fold normal as compared to the literature reports of 3.7 times fold normal (Figure 1E). Blood serum enzyme assays showed a distinct presence of β-gal in the serum until the 48-hour time point (Figure 1F). X-gal stains demonstrated wild type mice have bright blue coloring throughout all sections of the brain and spinal cord. Untreated mutant mice sacrificed at 8 weeks of age showed no presence of blue coloring in any neural tissue. Mutant mice treated with ApoE polymersomes had brain slices that had restored blue coloring after X-gal staining demonstrating that β-gal successfully reached and treated neural tissues.
Conclusions: Cy5 loaded ApoE polymersomes illustrate increased fluorescence in the brains of mutant mice as compared to wild type mice likely due to the upregulation of the LDLRs in states of neurological inflammation. Colocalization of Cy5 antibodies with neurons demonstrate effective transcytosis across the BBB. The blood serum fluorescence profile demonstrates that ApoE functionalized polymersomes remain in tissues for extended periods of time compared to the untargeted polymersomes. Enzyme assays show that β-gal reaches neurological tissues when delivered by ApoE polymersomes and still holds successful activity. Decrease in secondary enzyme pathways also confirms successful restoration of enzyme activity and degradation of the polymersome system. Similarly, we demonstrated that β-gal activity in the serum remains consistent until 48 hours where it is hypothesized to have undergone transcytosis to various tissues. Qualitative colorimetric assays support the conclusion that enzyme activity is restored to neurologic tissues using these functionalized polymersomes.