2025 AIChE Annual Meeting
(653b) Design and Engineering of PpL1 for Sensitive Detection of Antigens in Lateral-Flow Immunoassays Using Rabbit Single-Chain Fv Antibodies
Authors
Yoichi Kumada - Presenter, Kyoto Institute of Technology
Yodai Yamamoto, Kyoto Institute of Technology
Ngoc Minh Nguyen, Kyoto Institute of Tehcnology
Jun-ichi Horiuchi, Kyoto Institute of Technology
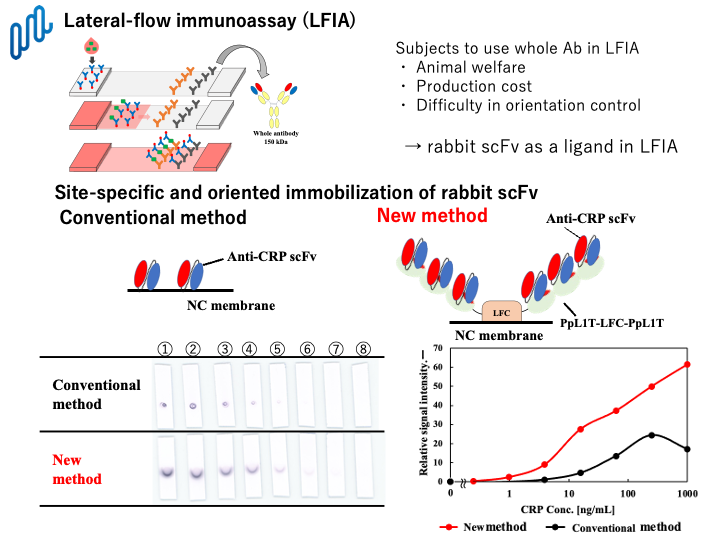
In this study, we have successfully developed a new and sensitive lateral-flow immunoassay for detection of antigen using bacterial PpL1 fusion protein and rabbit single-chain Fv antibodies. PpL1 is a single Ig-binding domain of Peptostreptococcus magnas protein L which is a highly thermostable and water-soluble protein. We have designed and demonstrated to produce PpL1-fusion proteins for site-directed immobilization of rabbit single-chain Fv antibodies. Among them, PpL1-LFC which is a fusion protein composed of PpL1 and C-terminal domain of bovine lactoferrin, maintained both Ig-binding activity and high adsorption ability toward nitrocellulose. Consequently, antigen of C-reactive protein as a model was successfully detected by scFv-based nitrocellulose membrane that rabbit scFv was indirectly immobilized on the surface of nitrocellulose membrane by site-specific interaction with PpL1-LFC. Tandem multimerization of PpL1 domain in PpL1-LFC resulted in enhancement of binding capacity for rabbit scFvs, and consequently, more than ten-fold lower LOD could be attained by this method, compared with that of the conventional lateral-flow immunoassay. Furthermore, point mutations of PpL1 could change the binding specificity towards rabbit scFvs and human IgGs. Consequently, this method was available even in the presence of serum proteins such as human IgGs. Antigen-binding specificities of rabbit scFvs can be easily changeable by our original CDR-grafting technology. Therefore, it would be expected that this method can be applicable to LFIAs for a variety of antigens, and contribute to obtain desirable signal intensities. Thus, our newly-developed LFIA method using PpL1-fusion protein and rabbit scFvs enable to improve detection signals to the greatest extent and applicable to those for a variety of antigen in clinical diagnostic tests.