2025 AIChE Annual Meeting
(305c) A Comprehensive Multiphysics Model for Full-Cycle Analysis of Thermochemical Hydrogen Production Redox Reactors
Authors
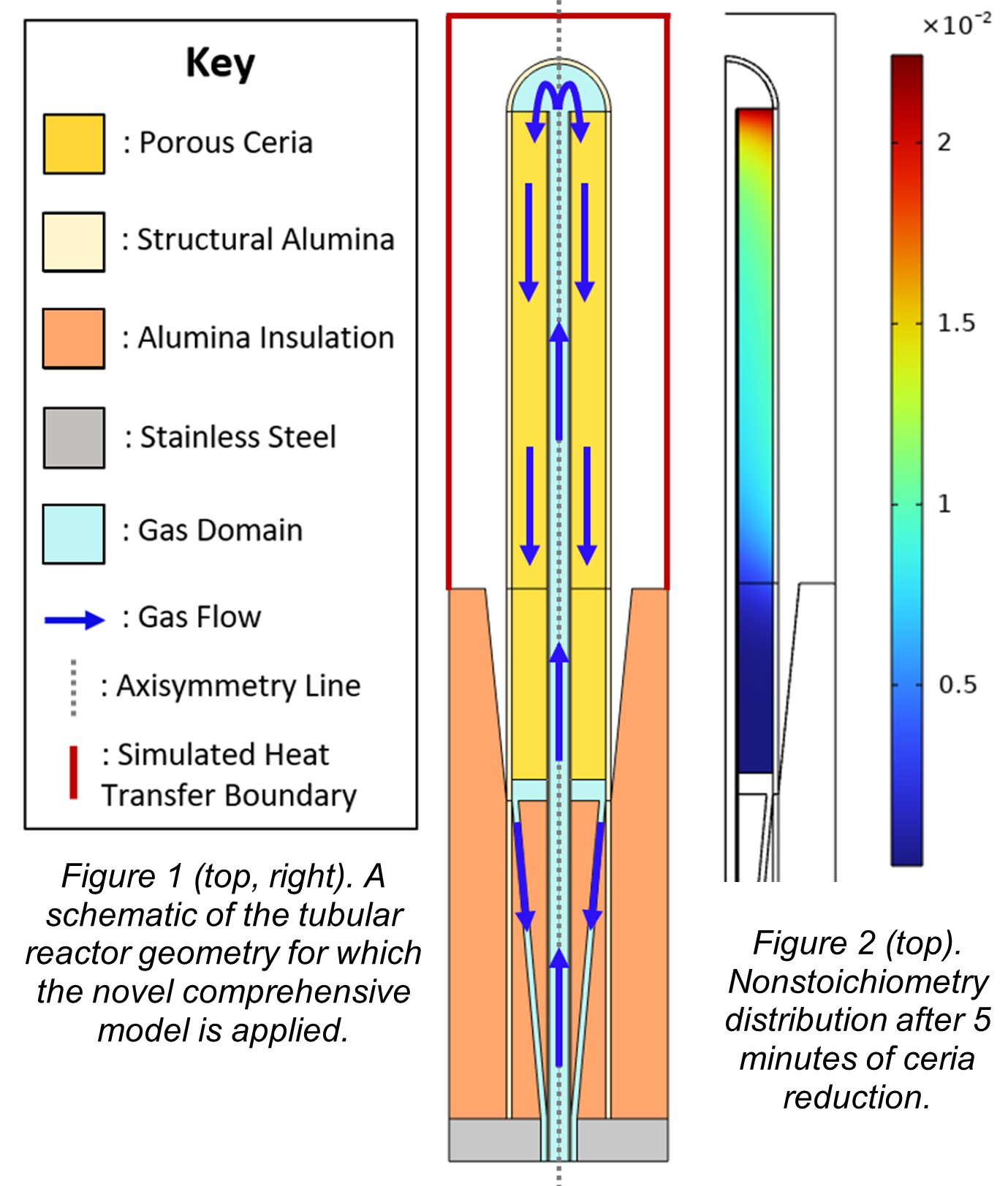
In previous work, we presented an approach for using apparent kinetic data to train an intrinsic kinetic law that can be integrated into numerical reactor models, and validated the model against experimental data on water splitting over nonstoichiometric ceria [1]. This type of kinetics treatment is often addressed to describe a range of reactions in the field of chemical looping and solar thermochemical processes, but a rigorous analytical methodology for the use of such kinetics in reactor modeling environment has not been widely adopted [3]. Here, we introduce a higher fidelity version of the multiphysics model where the hydrogen production rate equation is calibrated on the porous medium morphology, and we apply this comprehensive approach to describe the full cycle of a novel thermochemical hydrogen reactor. The approach is implemented in COMSOL Multiphysics®. Our high-fidelity model combines heat transfer, including radiation and local thermal non-equilibrium in the porous structure, fluid flow, reactive species transport, and chemical kinetics during both the cycle steps. The target reactor is a multi-tubular design that is being developed for use in the Reactor Train System which employs counterflow radiative heat exchange and indirect heating [4]. A schematic of this novel design is shown in Figure 1. Using a 2D axisymmetric geometry to model one of the reactor tubes, we simulate the entire redox cycle while varying parameters such as the tube length, redox material porosity, vacuum level upon reduction, and flow rates. For sake of example, Figure 2 depicts the nonstoichiometry distribution after only 5 minutes of reduction using ceria as the redox material during a typical run. Moreover, since the tubular reactor is designed to operate with different redox materials, we analyze both state-of-the-art ceria as well as a novel perovskite. One of our objectives is to demonstrate that the model works for materials with vastly different redox thermodynamic and kinetic properties, when available in the literature. Our results elucidate the impact of the oxidation kinetics on the cycle performance and highlight the importance of using accurate modeling for this purpose. A parametric study will be employed for the optimal design and operation of a prototype Multi-Tubular Reactor.
[1] F. Orsini, D. Ferrero, D. Papurello, and M. Santarelli, “Solar Thermochemical Fuel Production: A Novel, Validated Multiphysics Reactor Model for the Reduction–Oxidation of Nonstoichiometric Redox Cycles,” Energies, vol. 18, no. 2, Art. no. 2, Jan. 2025, doi: 10.3390/en18020414.
[2] A. De La Calle, I. Ermanoski, J. E. Miller, and E. B. Stechel, “Towards chemical equilibrium in thermochemical water splitting. Part 2: Re-oxidation,” Int. J. Hydrog. Energy, vol. 72, pp. 1159–1168, Jun. 2024, doi: 10.1016/j.ijhydene.2024.05.298.
[3] V. M. Wheeler, R. Bader, P. B. Kreider, M. Hangi, S. Haussener, and W. Lipiński, “Modelling of solar thermochemical reaction systems,” Sol. Energy, vol. 156, pp. 149–168, Nov. 2017, doi: 10.1016/j.solener.2017.07.069.
[4] A. S. Patankar, X.-Y. Wu, W. Choi, H. L. Tuller, and A. F. Ghoniem, “A Reactor Train System for Efficient Solar Thermochemical Fuel Production,” in Volume 8B: Energy, Virtual, Online: American Society of Mechanical Engineers, Nov. 2021, p. V08BT08A024. doi: 10.1115/IMECE2021-69716.