2025 AIChE Annual Meeting
(345e) Biosynthesis of Isonitrile Lipopeptide Metallophores from Pathogenic Mycobacteria
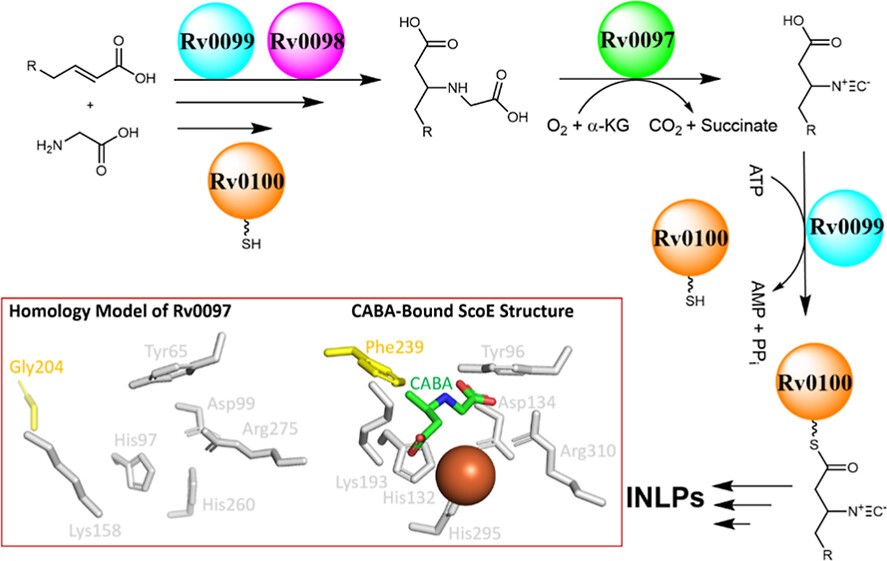
Isonitrile lipopeptides (INLPs) are known to be critical to the virulence of pathogenic mycobacteria by mediating metal transport, but their biosynthesis remains obscure. In this work, we use in vitro biochemical assays, structural biology, site-directed mutagenesis, chemical synthesis, and spectroscopy techniques to scrutinize the activity of core enzymes required for INLP biosynthesis in mycobacteria. Compared to environmental Streptomyces, pathogenic Mycobacterium employ a similar chemical logic and enzymatic machinery in INLP biosynthesis, differing mainly in the fatty-acyl chain length, which is controlled by multiple enzymes in the pathway. Our in-depth study on the non-heme iron(II) and α-ketoglutarate-dependent dioxygenase for isonitrile generation, including Rv0097 from Mycobacterium tuberculosis (Mtb), demonstrates that it recognizes a free-standing small molecule substrate, different from the recent hypothesis that a carrier protein is required for Rv0097 in Mtb. A key residue in Rv0097 is further identified through homology-based modeling to dictate the varied fatty-acyl chain length specificity between Streptomyces and Mycobacterium through enzyme engineering efforts. Finally, we present high-resolution co-crystal structures of Rv0097 with several substrates, laying the groundwork for elucidating the molecular mechanism behind isonitrile formation. A fundamental understanding of the isonitrile-forming mechanism also provides strong support for exploring Rv0097 as a potential drug target in the battle against mycobacterial infections, including tuberculosis, which causes millions of deaths each year.