2025 AIChE Annual Meeting
(473b) Biohybrid Helix–Macrophage Magnetic Microrobots for Cellular Transport through Mucus and Dispersion over Boundaries
Authors
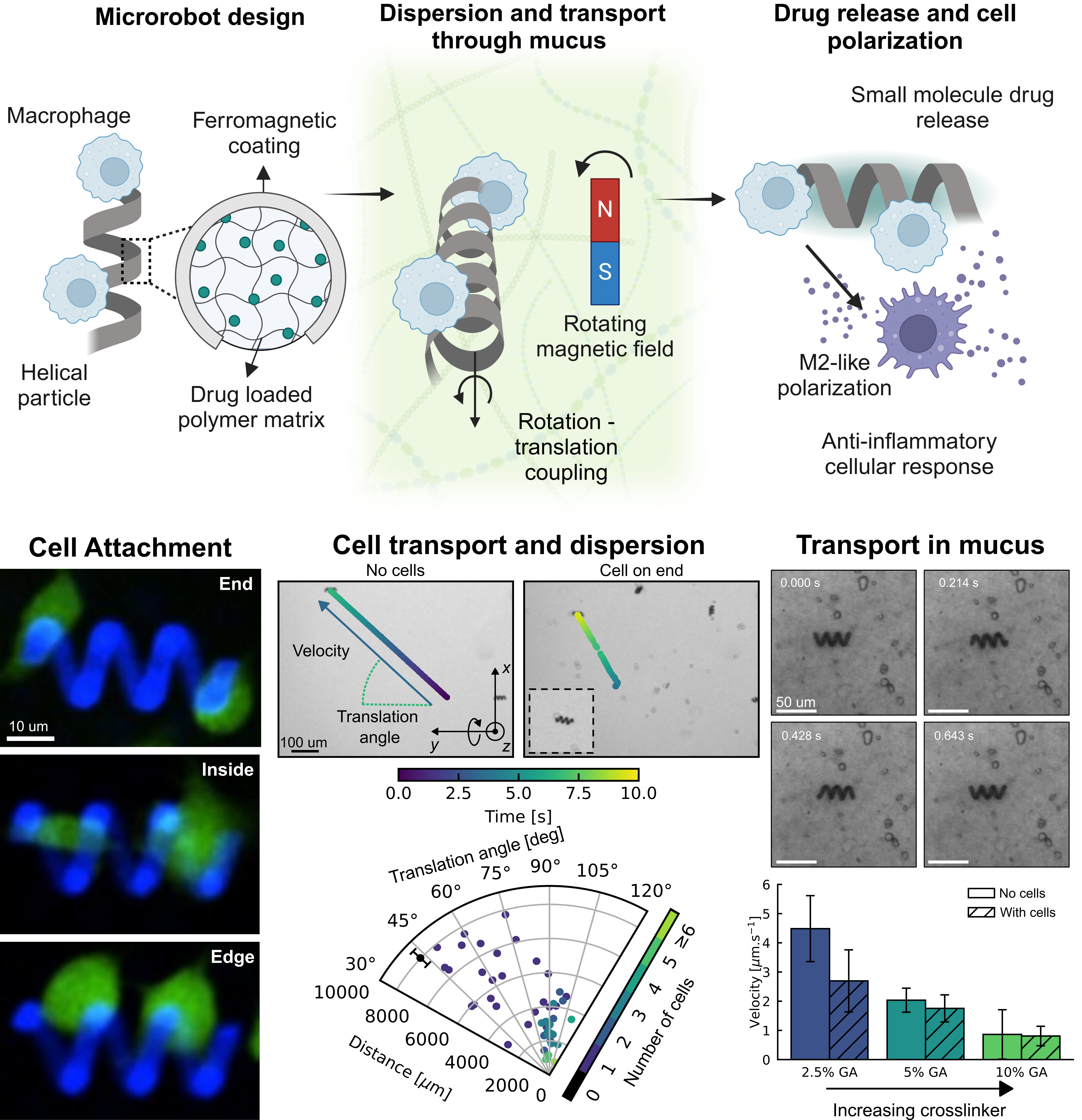
Helical microrobots, 50 microns in size, were fabricated with two-photon lithography and coated with chromium, nickel, and titanium to endow magnetic responsiveness in a biocompatible shell. RAW 264.7 murine macrophages were added to the helices, and the spatial distribution of cell attachment was characterized using confocal microscopy. After, we studied the locomotion of the cell/helix complexes in a rotating magnetic field. We found that the attachment location and number of cells attached had a deterministic effect on locomotion. We were able to probe the physical mechanisms for these effects through a microhydrodynamic mobility framework. An interesting consequence of cell attachment was that cell/helix complexes disperse over a larger area than the helices alone. This may be beneficial for the treatment of diseased tissues. Following this we examined the ability of the complexes to transport through physiologically representative mucus. We found that despite the increased viscosity of mucus, the complexes were still able to transport through mucosal environments under magnetic actuation.
We concluded by demonstrating proof-of-concept therapeutic applicability. We loaded a small molecule drug into the polymeric bodies of the helical microrobots, characterized drug release, and showed that macrophage polarization can be guided in response to the released drug. Our work illustrates that magnetic helical microrobots can enhance the transport and function of living cells (e.g., immune cells, gene-altered cells) as therapies through viscous fluids for potential clinical use.