2025 AIChE Annual Meeting
(640h) AI-Guided Catalyst Design for Selective Acetate Production in CO Electroreduction
Authors
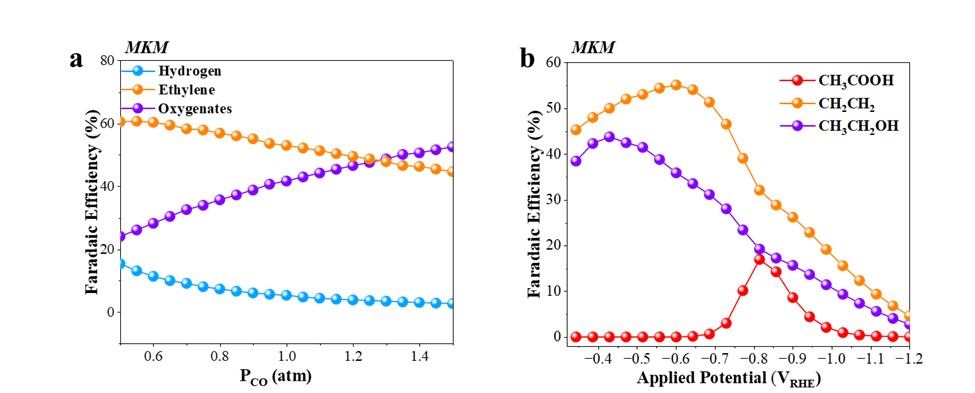
In this work, we present a multi-scale modeling framework combining grand-canonical density functional theory (GC-DFT) and microkinetic modeling (MKM) to uncover reaction pathways and rate-determining steps (RDS) for CORR toward key products: hydrogen, ethylene, ethanol, acetate, CO, and methane. Our model predictions (Fig. 1a-b) show product distributions as functions of CO partial pressure and applied potential. We identify that acetate formation proceeds through CO–CHₓ coupling, whereas ethylene and ethanol form via CO–CHO pathways. Notably, CH* binding energy emerges as a predictive descriptor for acetate selectivity, exhibiting a volcano relationship.
Building on mechanistic understanding, we leveraged advanced active learning algorithms to guide catalyst discovery and optimization within a vast design space of Cu-based systems. This data-driven approach rapidly identified several alloy compositions predicted to outperform pure Cu. Experimental validation in electrochemical setups further supported these findings, highlighting the potential of AI-assisted methodologies in accelerating catalyst development. Overall, this integrated computational-experimental framework offers a powerful tool for navigating complex catalytic landscapes and advancing next-generation electrochemical processes.
Fig.1. Faradaic efficiencies (FEs) versus CO partial pressure at –0.5 VRHE; (b) FEs versus applied potential at PCO= 1 atm.
References
(1) Liu, S.Q. Int. J. Food Microbiol. 2003, 83 (2), 115–131.
(2) Kiefer, D. et al., Trends Biotechnol. 2021, 39 (4), 397–411.