2025 AIChE Annual Meeting
(227c) 3D Printed Self-Powered Piezoelectric Smart Scaffolds for Enhanced Bone Tissue Regeneration
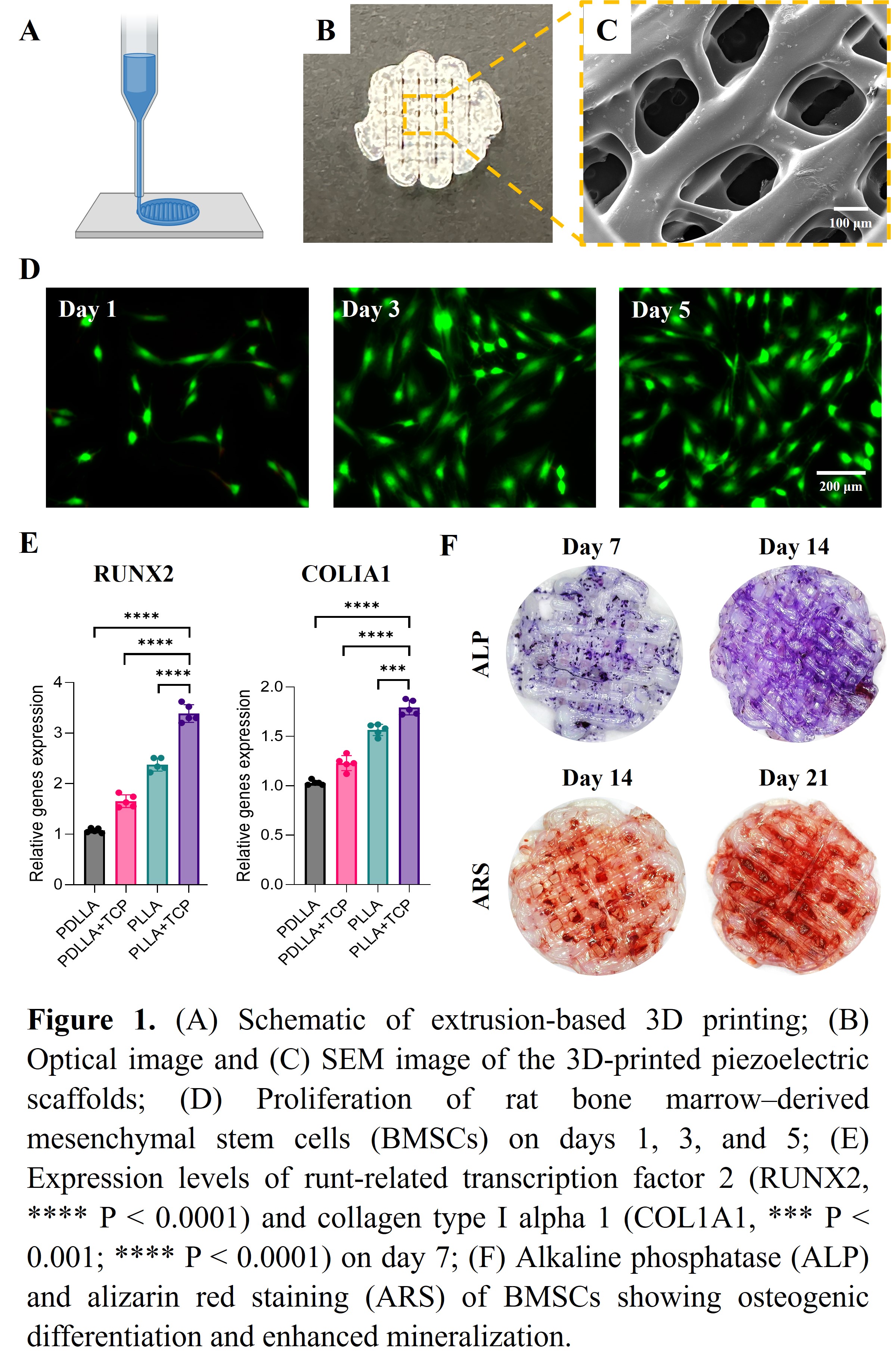
In vitro studies demonstrated that rat bone marrow–derived mesenchymal stem cells (BMSCs) exhibited enhanced osteogenic differentiation when cultured on the piezoelectric smart scaffolds under ultrasound stimulation. Cell proliferation progressively increased on days 1, 3, and 5 (Figure D). After 7 days of ultrasound stimulation, qPCR analysis revealed upregulated expression of key osteogenic genes, including runt-related transcription factor 2 (Runx2), collagen type I alpha 1 (COLIa1), and alkaline phosphatase (ALP) (Figure E). Alkaline phosphatase activity was significantly elevated at both 7 and 14 days, indicating early osteoblast lineage commitment. At later stages, alizarin red staining (ARS) demonstrated extensive calcium deposition and mineralized nodule formation at 14 and 21 days, confirming enhanced extracellular matrix mineralization (Figure F). Current research involves assessing the 3D-printed piezoelectric smart scaffolds' long-term bioactivity in promoting important regenerative processes including angiogenesis and cell migration, as well as how well they function in a model of calvarial defects.
In summary, 3D-printed piezoelectric smart scaffolds exhibited enhanced piezoelectric responsiveness and biological activity, thereby promoting osteogenesis both in vitro and in vivo. This study demonstrates that the integration of piezoelectric scaffolds with ultrasound stimulation provides a self-powered and bioactive platform, offering great promise for accelerating bone regeneration in orthopedic defects.