Introduction: Fetal growth restriction (FGR) occurs when a fetus is unable to grow normally due to inadequate nutrient and oxygen supply. Globally, around 32 million babies are born growth restricted and FGR is a leading cause of perinatal death and long-term disability. The brain is particularly vulnerable to prolonged hypoxic FGR conditions, and adverse outcomes cause life-long complications that range from learning and behavioral issues to cerebral palsy. It is vital to treat FGR newborns early to prevent or reduce life-long disorders. However, there are no treatments to protect the FGR newborn brain. Inflammation is associated with brain impairment in the FGR newborn, resulting in sustained activation of pro-inflammatory microglia that persists up to weeks after birth in the FGR pig brain and in the blood of FGR infants. Curcumin is an anti-inflammatory and antioxidant chemical, which is widely used to treat oxidative and inflammatory conditions, metabolic syndrome, arthritis, anxiety, and hyperlipidemia, and has been demonstrated had neuroprotection effects in neonatal rats. However, the bioavailability of curcumin is greatly affected by its low solubility. Nanoparticles can encapsulate curcumin and improve its solubility, yet the encapsulation efficiency is limited and often less than 10% by weight of the drug-polymer formulation. To optimize the curcumin encapsulation efficiency in poly(lactic-co-glycolic acid)-poly(ethylene glycol) (PLGA-PEG) nanoparticles, we investigated the effects of PLGA length, stabilizer concentration, polymer functionalization, and formulation method on curcumin loading. Upon successful optimization of a PLGA-PEG nanoparticle with high curcumin encapsulation efficiency and drug loading we also sought to assess particle accumulation in the FGR piglet brain to evaluate its potential for FGR treatment.
Methods: PLGA-PEG with 15k, 45k, 75k and 70k-70k PLGA length, stabilizer Pluronic® F127 (F127) with concentration at 0.1% (w/v), 0.5% (w/v), 1% (w/v) and 10% (w/v), PLGA-PEG with amine or NHS-curcumin functionalization, and both standard nanoprecipitation and sequential nanoprecipitation formulation methods were iterated to identify an optimal curcumin formulation with high drug loading, reproducibility, and long-term stability. After establishing the optimal curcumin-loaded nanoparticle (NanoCurc), fluorescent dye CF555® and CF647® were conjugated to PLGA-PEG backbone, followed by sequential nanoprecipitation technique to formulate dye-labeled NanoCurc (CF555-NanoCurc and CF647-NanoCurc). FGR is a spontaneously occurring model in piglets. Newborn FGR (<10th percentile) and normally grown (NG) piglets (n=7) were administered 10 mg/kg CF555-NanoCurc intravenously (i.v) and 10 mg/kg CF647-NanoCurc intranasally (i.n.) on postnatal day 1 (P1). At 4 h and 48 h after administration, blood was collected, and pigs were sacrificed to collect the brain, liver, spleen, and kidney. Brain sections were prepared from the parietal cortex and the cerebellum and stained with anti-NeuN (neuron marker) and anti-Iba1 (microglia marker), in addition to a nuclear stain. Images were acquired using a confocal microscope to analyze NanoCurc uptake and localization in the FGR and NG piglet brain.
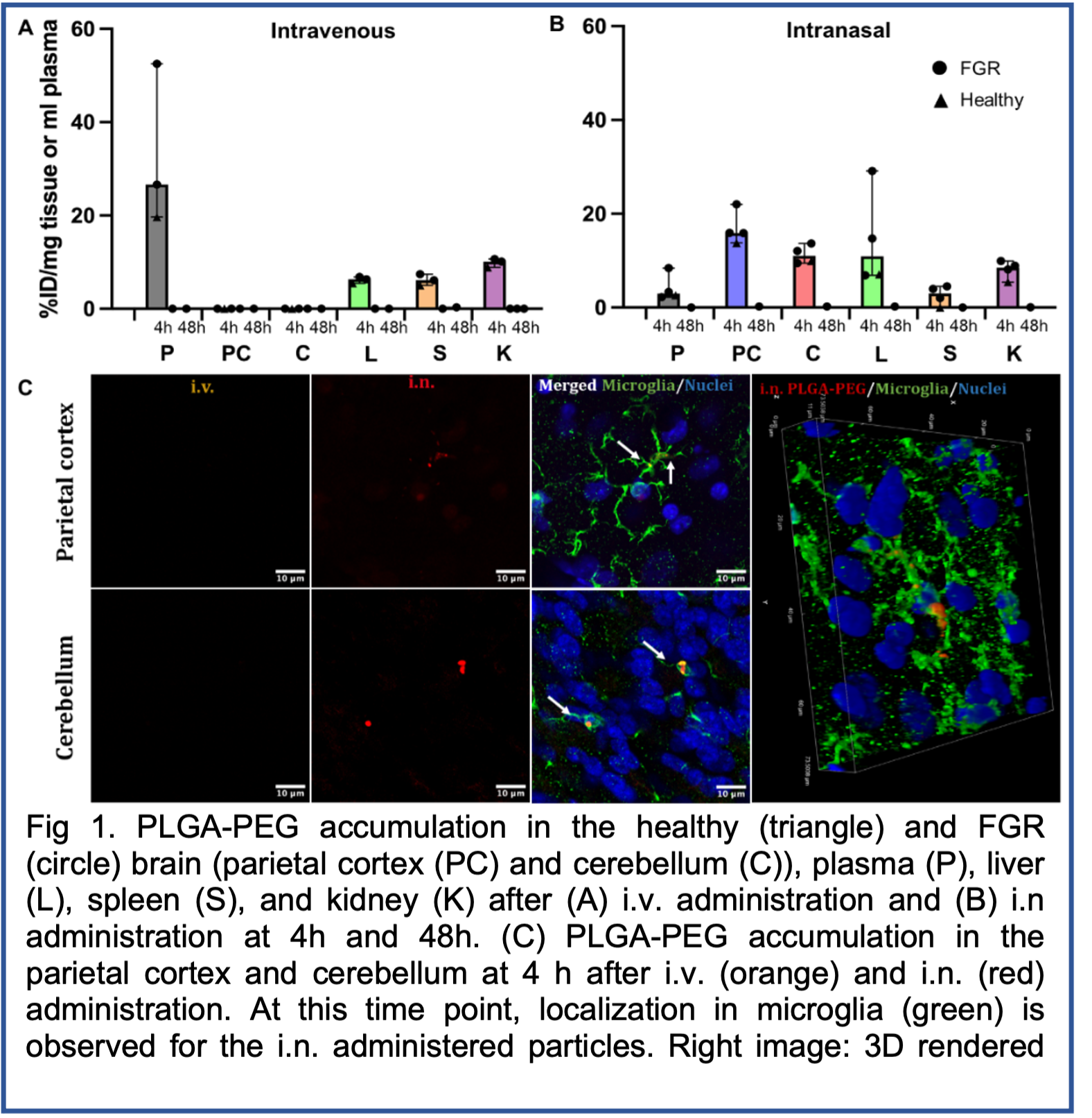
Results: We found that the size of curcumin nanoparticles increased with the increase of PLGA length and the decrease of the F127 concentration. The curcumin loading reached the highest when formulated with 45k PLGA-PEG. Also, curcumin loading increased when F127 concentration decreased, although the stability and reproducibility of curcumin-loaded nanoparticles were limited when F127 concentration was 0.1%. PLGA-PEG functionalization could partially improve curcumin loading, but undermined the lyophilization stability and caused issue for long-term storage. Curcumin-loaded nanoparticles formulated with standard nanoprecipitation had less than 10% curcumin loading because of the different precipitation rates. We identified that sequential nanoprecipitation resulted in higher drug loading compared to nanoprecipitation. Our optimal NanoCurc formulation was 50 to 60 nm hydrodynamic size, a near neutral surface charge, high drug loading (38%) and drug encapsulation efficiency (>95%), and long-term (at least 6 months) stability at room temperature. The i.n. (Fig. 1B) administration resulted in 2-fold higher particle % injected dose (ID) in the brain parietal cortex and cerebellum at 4 h after administration compared to particles administered i.v. (Fig 1A). Particles were colocalized in microglia as early as 4 h after i.n. administration (Fig. 1C). At 48 hours post treatment particle accumulation decreased to almost undetectable levels. Off-target accumulation in the liver, spleen, and kidney were quantified, with under 10% ID accumulated in these organs at 4 h, and undetectable NanoCurc at 48 h after administration.
Conclusion: With sequential nanoprecipitation, a reproducible NanoCurc formulation with high drug loading and long-term stability can be formulated. The NanoCurc formulatoin reflects a promising platform to deliver a neuroprotective substance to the FGR brain. Our biodistribution study in piglets also demonstrates the validity of intranasal administration of nanoparticles to rapidly reach the newborn brain. These data indicate i.n. administration may be a more favorable route for brain delivery compared to i.v. administration.