Introduction: Glioblastoma Multiforme (GBM), an aggressive, damaging and heterogenous form of brain tumor is lethal and incurable. Existing therapeutic strategies include surgical resection, chemotherapy, external radiotherapy and/or combinations thereof
1. However, therapeutic efficacy
in vivo is limited mainly because of three reasons: (1) the location of tumors, that make frequent and aggressive therapies difficult without affecting the surrounding healthy brain, (2) the development of resistance by tumor cells to existing treatments, and (3) heterogenous intratumoral distributions and/or limited permeability of therapeutics across the blood brain tumor barrier
2,3. With a mean survival of only about 8 – 15 months after diagnosis, and a 5 – year survival rate of hardly 10%
4,5, there is an urgent unmet need for selective and effective treatment of GBM tumor cells to effect potent and durable tumor cell kill and to provide long-lasting remission.
We investigated a systemically injected ‘cocktail’ of alpha-particle RadioPharmaceutical Therapeutics (α-RPT) with low-doses of a DNA-damaging, standard-of-care chemotherapeutic, temozolomide (TMZ). α-RPT is a form of internal radiotherapy that deposits massive amounts of energy by highly charged α-particles, which produce complex double-strand DNA breaks, as they traverse tissue, leading to cell kill independent of the cell’s oxygenation state and/or resistance to other agents. With their short range of 5 – 10 cell diameters, α-particles allow for localized irradiation of target tumor cells with minimal toxicity to peripheral healthy cells. However, only cells being hit by α-particles will be killed 6,7. Therefore, the heterogeneous intratumoral distributions of the carriers of α-particles limit efficacy.
For the delivery of the alpha-particle emitter actinium-225 we utilized dendrimers (~ 6-7 nm) labeled with actinium-225 (nano-radiotherapy) that (1) can penetrate the blood brain tumor barrier upon systemic administration owing to their nanosize, but do not penetrate the blood brain barrier of the healthy tumor; (2) they extensively associate with tumor-promoting tumor associated macrophages (TAMs) that infiltrate the tumor microenvironment, allowing for the α-emitters to uniformly irradiate brain tumors at the site of cancer-induced inflammation; and, importantly, (3) they quickly clear from the body when not associated with TAMs, thereby decreasing off-target toxicities 8,9. When combined with low, non-lethal doses of chemotherapy, we unexpectedly observed improved therapeutic efficacy, compared to radiotherapy alone attributed to (1) the increased tumor uptake of alpha-particle nano-radiotherapy, and (2) the enhanced killing potential when both modalities acted on same cancer cells.
Materials and Methods: In vitro, the uptake of dendrimers by M1, M2 activated and resting macrophages, and glioblastoma GL261 cells was determined. The sensitivity and survival fraction (SF) of GL261 cells to 225Ac-DOTA,225Ac-radiolabeled dendrimer nanoparticles (225Ac-D), TMZ and the combination ‘cocktail’ at the physiological pH 7.4 using 2D clonogenic survival assays was assessed 10. The correlation of SF to radioactivity per cell uptake was investigated. To assess the efficacy of the proposed therapeutic, 3D multicellular spheroids of GL261 cells (analogues of the tumor avascular regions) simulating the acidic tumor microenvironment were developed and the extent of cell regrowth after exposure to 225Ac-D and TMZ as single and combination treatments were evaluated. In vivo, C57BL/6 mice inoculated with intracranial GL261 tumors were employed to evaluate efficacy. The biodistributions and dosimetry in mice exposed to 225Ac-D with and without TMZ were determined.
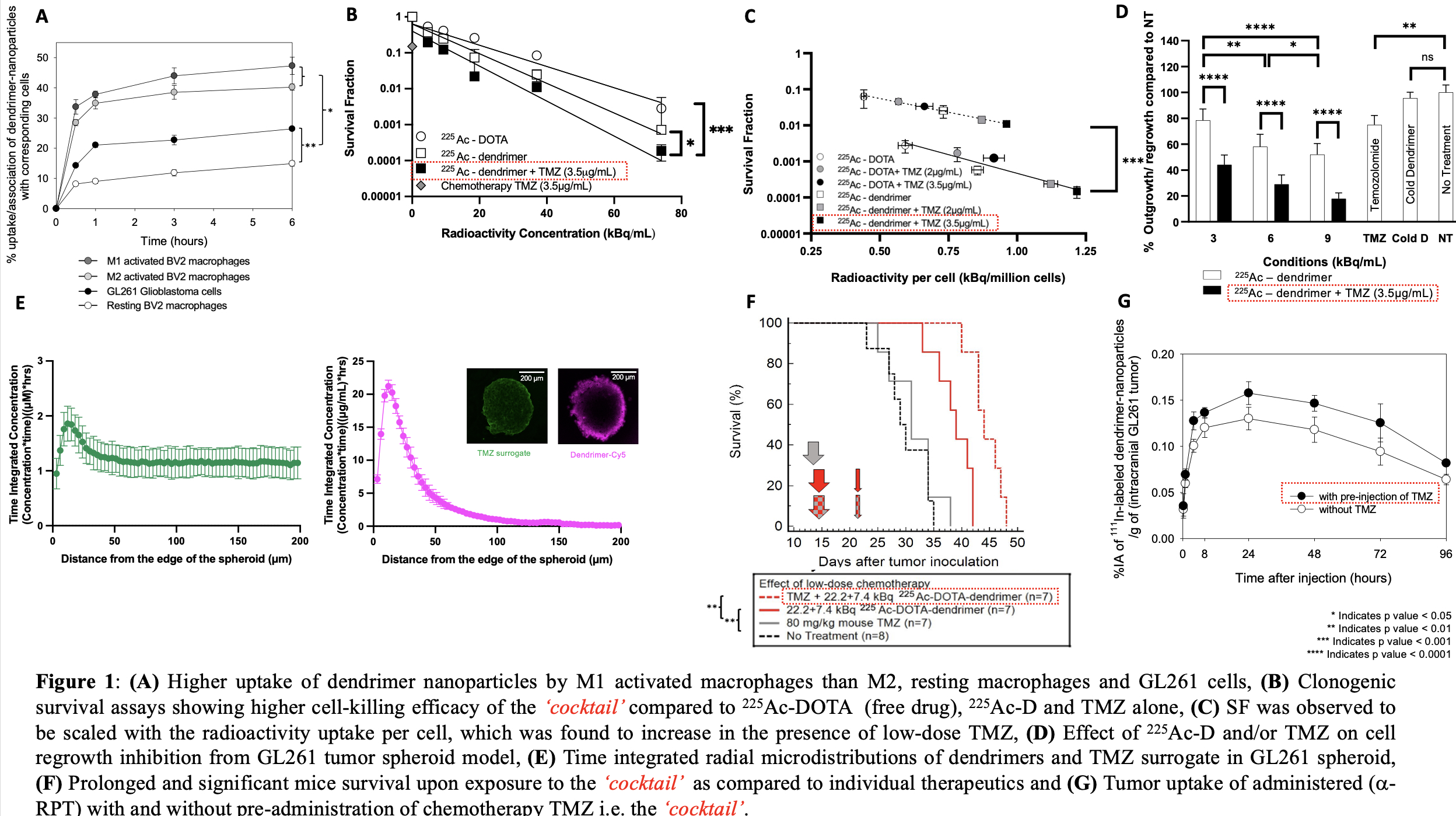
Results and Discussion: The uptake of dendrimers (Figure 1A) by M1 activated macrophages was significantly higher than M2, resting macrophages and/or GL261 glioma cells. Clonogenic survival assays indicated lower cell survival when exposed to the ‘cocktail’ than 225Ac-D and/or TMZ alone (Figure 1B). SF was observed to be scaled with the radioactivity uptake per cell, which was found to increase in the presence of low-dose TMZ (Figure 1C). In 3D spheroids formed by the same GL261 cell line, the extent of spheroid regrowth inhibition after exposure to the ‘cocktail’ was more pronounced compared to each of the independent therapies alone (Figure 1D), independent of the interstitial acidification known to affect TMZ efficacy. The time-integrated radial concentrations of dendrimers and TMZ demonstrated limited penetration of dendrimers due to their association with GL261 cells and deep penetration of the TMZ surrogate (Figure 1E). In vivo, prolonged mouse survival (48 days) was observed for the ‘cocktail’ in comparison to no treatment (35 days), TMZ (38 days) and/or 225Ac-D alone (42 days) (Figure 1F). Low-dose TMZ treatment increased tumoral uptake of 225Ac-D injected later with minimal off-target toxicities (Figure 1G).
Conclusions: In this study, we observed remarkable efficacy in prolonging survival, without off-target toxicities, of immune competent mice with intracranial syngeneic glioblastoma when combining low, non-lethal doses of standard-of-care chemotherapy and tumor-infiltrating αRPT compared to each modality alone.
References:
1. Anjum K, Shagufta BI, Abbas SQ, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomedicine and Pharmacotherapy. 2017;92:681-689. doi:10.1016/j.biopha.2017.05.125
2. Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. Journal of Experimental and Clinical Cancer Research. 2022;41(1). doi:10.1186/s13046-022-02349-7
3. Jackson C, Westphal M, Quiñones-Hinojosa A. Complications of glioma surgery. In: Handbook of Clinical Neurology. Vol 134. Elsevier; 2016:201-218. doi:10.1016/B978-0-12-802997-8.00012-8
4. Davis ME. Glioblastoma: Overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5):1-8. doi:10.1188/16.CJON.S1.2-8
5. Aldoghachi AF, Aldoghachi AF, Breyne K, Ling KH, Cheah PS. Recent Advances in the Therapeutic Strategies of Glioblastoma Multiforme. Neuroscience. Published online 2022. doi:10.1016/j.neuroscience.2022.03.03
6. Sofou S. Radionuclide Carriers for Targeting of Cancer. Vol 3.; 2008.
7. McDevitt MR, Sgouros G, Sofou S. Targeted and Nontargeted α-Particle Therapies. Annu Rev Biomed Eng. 2018;20:73-93. doi:10.1146/annurev-bioeng-062117-120931
8. Liaw K, Zhang F, Mangraviti A, Kannan S, Tyler B, Kannan RM. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng Transl Med. 2020;5(2). doi:10.1002/btm2.10160
9. Sharma A, Liaw K, Sharma R, et al. Dendrimer-Mediated Targeted Delivery of Rapamycin to Tumor-Associated Macrophages Improves Systemic Treatment of Glioblastoma. Biomacromolecules. 2020;21(12):5148-5161. doi:10.1021/acs.biomac.0c01270
10. Ranjit Nair R, Prasad A, Bhatavdekar O, Sarkar A, Gabrielson KL, Sofou S. Combined, yet Separate: cocktails of carriers (not drugs) for α-particle therapy of solid tumors expressing moderate-to-low levels of targetable markers. Eur J Nucl Med Mol Imaging (2024) accepted.