2024 AIChE Annual Meeting
(184q) Development of a Lab-Scale Fluid Bed Granulation Process within a Semi-Continuous Manufacturing Workflow
Authors
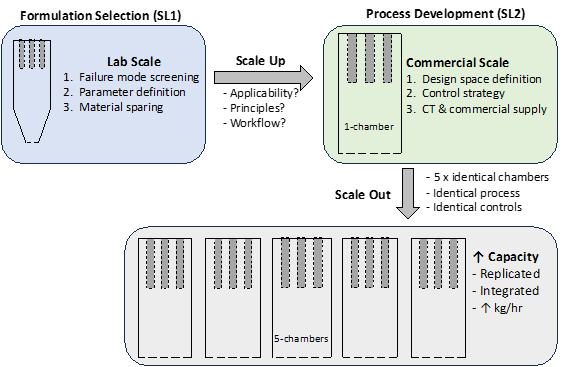
Fluid bed wet granulation (FBG) is a common pharmaceutical unit operation where a powder blend is fluidized, sprayed with a binder solution, and dried within a single process vessel. The purpose of including a granulation step in a drug product manufacturing process is to improve physical properties of an active pharmaceutical ingredient (API) by agglomerating polydisperse particles into a uniform size distribution. While FBG is typically operated as a batch process, semi-continuous manufacturing processes have recently emerged which offer a hybrid option offering the well-established advantages of both batch and continuous processing techniques [1]. Recently, a semi-continuous fluid bed granulation system has been introduced commercially (Xelum®, Syntegon, Germany), with broadly three iterations for consideration (Figure 1): lab-scale (SolidLab 1 -SL1), commercial scale 1-chamber (Xelum R&D), and a commercial scale 5-chamber. The SL1 is envisioned as a material sparing lab-scale benchtop fluid bed granulator for formulation prototyping and process parameter screening. The 1-chamber SolidLab 2 (SL2) is envisioned for at-scale process development, clinical trial and initial commercial production. The 5-chamber is positioned as a large-scale commercial manufacturing platform.
In pharmaceutical development, implementation of a lab-scale FBG for early formulation development poses several advantages. First, it allows for the reduction of total API used during development which is often a limiting resource. Second, lab-scale FBG may be used to evaluate prototype formulations for CQA screening. Finally, process and formulation variables may be characterized via appropriate experimental designs, while reducing time and material investment.
The objective of this work is to develop a comprehensive process understanding of a lab-scale FBG. First, a representative batch size was established utilizing prior experience, vendor recommendations, and preliminary ranging studies. The lab-scale FBG was further characterized at the established batch size by a statistical design of experiments (DOE) based approach to investigate the impacts of formulation attributes, operational parameters, and design variables on relevant desired quality attributes of granules and tablets. In addition, insights gained from the DOE were used to evaluate the feasibility of a dimensionless scaling criterion, the evaporative energy to drying capacity (EE/DC) ratio [2], to maintain granule characteristics across granulation scales (lab to commercial). Finally, a multiway partial least squares model (MPLS) was constructed to characterize the dynamic impact of process variables on granule characteristics.
2) Methods
This study delves into four APIs (micronized Acetaminophen or APAP, LY-1, LY-2, and LY-3) distinguished by their distinct particle sizes, bulk densities, and flowabilities. These APIs were selected for this analysis to demonstrate process robustness across a range of typical material-intrinsic failure modes observed at high drug load formulations for a direct compression process. Based on previous prototyping evaluations, formulations containing a high drug load of LY-1 and compressed via direct compression (DC) produced tablets with low tensile strengths. In addition, formulations containing high drug loads of LY-2 demonstrated poor flow and the corresponding tablets showed edge chipping and picking/sticking. Finally, LY-3 demonstrated prototyping challenges with poor flow and corresponding tablet weight variation when processing via DC or roller compaction (RC). Each formulation contained a drug load greater than or equal to 40%, a filler (MCC), a binder (HPC), and a disintegrant (CCS). Small amounts of extragranular excipients (Syloid, SSF, or MgSt) were added as needed (<6.5%).
Granulation operating parameters were varied in an initial ranging study to observe the failure modes of the FBG process. Once a successful operating space was defined, granules were tested for particle size distribution (PSD) to identify a target set of conditions that produced desirable granules representative of the commercial process. Granules were tested for PSD, bulk and tapped density, final moisture content, and flow properties (via shear cell testing).
The selected final blends were compressed into tablets using an HB50 compaction simulator (Huxley Bertrum Engineering Ltd., Cambridge, UK) simulating a Korsch XL200 rotary tablet press at 90 rpm. TSM B round flat face punches with a 6mm diameter were employed. For each selected final blend, three compression pressures were applied, resulting in tablets with different solid fractions. Tablets were tested for weight, thickness, and hardness measurements, as well as disintegration times.
3) Results & Discussion
A preliminary set of experiments was conducted to establish an initial operating space for further development. The goal of this work was to identify a value for EE/DC that produced a representative granulation at a 400 g batch size, such that the resulting granules possess similar characteristics to those from a typical commercial process. Subsequently, EE/DC was calculated for the successful runs and used for scaling down batch size to 200 g and 100 g. For a particular set of process conditions, this ratio indicates the amount of moisture in the granule bed during the granulation phase of an FBG process.
Granulations with 100 g batch sizes suffered from poor fluidization and an overall lack of reproducibility in granule size distribution. Thus, 200 g was selected as a minimum batch size for further work. Select runs at the 400 g and 200 g scale were evaluated for flowability and compactibility. Tablets from these granulations demonstrated good tensile strength (>2.0 MPa) and acceptable disintegration times (<5 minutes).
Next, three APIs (LY-1, LY-2, and LY-3) were granulated in place of micronized APAP in the formulation at the identified target conditions for the 200 g batch size. In all cases, the granules were blended with a lubricant (sodium stearyl fumarate) and compressed into tablets to evaluate the critical quality attributes. LY-1 tablets had an average tensile strength of 2.4 MPa at the nominal solid fraction (0.85). For LY-2, optical microscopy was performed on the compressed tablets to confirm that no edge chipping was observed and picking/sticking was significantly reduced. Upon comparison with previously made tablets, it was determined that the SL1 tablets resulted in better tablet compaction at high drug loads. Lastly, for LY-3, PSD results demonstrate acceptable particle size enlargement.
As a third objective, the SL1 process ranges for a 200 g batch size were defined to understand the quantitative effect of process (binder spray rate, inlet air temperature, spray air pressure) and design variables (nozzle air cap size) on granule attributes via DOE-based experimentation. For this study, extreme conditions were selected to simulate a high- and low-moisture accumulation/removal rate scenarios, which correspond to over-granulation and under-granulation conditions, respectively. Using the process data and granule responses captured from the 17-run DOE, a MPLS model was built using 4 latent variables and explains 90.8% of the variance in the granule size descriptors. Among all variables in the process data studied, the spray air pressure was found to have the highest correlation with the granule attributes.
4) Conclusion
In this study, a lab-scale fluid bed granulation process was evaluated at multiple process conditions, batch sizes and formulations. A dimensionless scaling criterion, EE/DC, was used to maintain granule characteristics across batch size. Three high drug load formulations containing different APIs (LY-1, LY-2, and LY-3) showed improvements in their respective failure modes when granulated in the SL1 prior to compression. Furthermore, a 17-run DOE was executed to characterize the impact of process and design variables on the granule attributes. Among the variables studied, the spray air pressure was found to have the most significant effect on the granule size descriptors.
Figure 1. Process development workflow for semi-continuous fluid bed granulation
5) References
[1] Muzzio, F., & Oka, S. (Eds.). (2022). How to Design and Implement Powder-to-Tablet Continuous Manufacturing Systems. Academic Press.
[2] E. Gavi and A. Dischinger, "Scale-up of Fluid Bed Granulation Using a Scale-Independent Parameter and a Process Model," AAPS PharmSciTech, vol. 22, no. 4, p. 148, May 5 2021, doi: 10.1208/s12249-021-02013-x.