Introduction: Postmenopausal osteoporosis (PMO) is a chronic inflammatory disease characterized by decreased bone mass and increased bone fracture risk. Estrogen deficiency during menopause plays a major role in PMO by influencing bone, immune, and gut cell activity [1, 2]. In the gut, estrogen loss decreases tight junction proteins that bind epithelial cells of the intestinal barrier together [3]. This increases gut permeability and alters the balance of T cells, increasing inflammatory T helper (Th17) cells and decreasing anti-inflammatory T regulatory cells (Tregs) [3]. Prebiotic dietary fibers are a promising modality to treat gut-mediated inflammation and bone loss associated with estrogen deficiency [4, 5, 6]. In a previous study, we investigated the effect of butyrate (a prebiotic by-product) on Treg levels in the gut, blood, and bone starting with healthy conditions [7]. However, a gap remains in our mechanistic understanding of how estrogen affects systemic inflammation separately and in combination with prebiotic fibers.
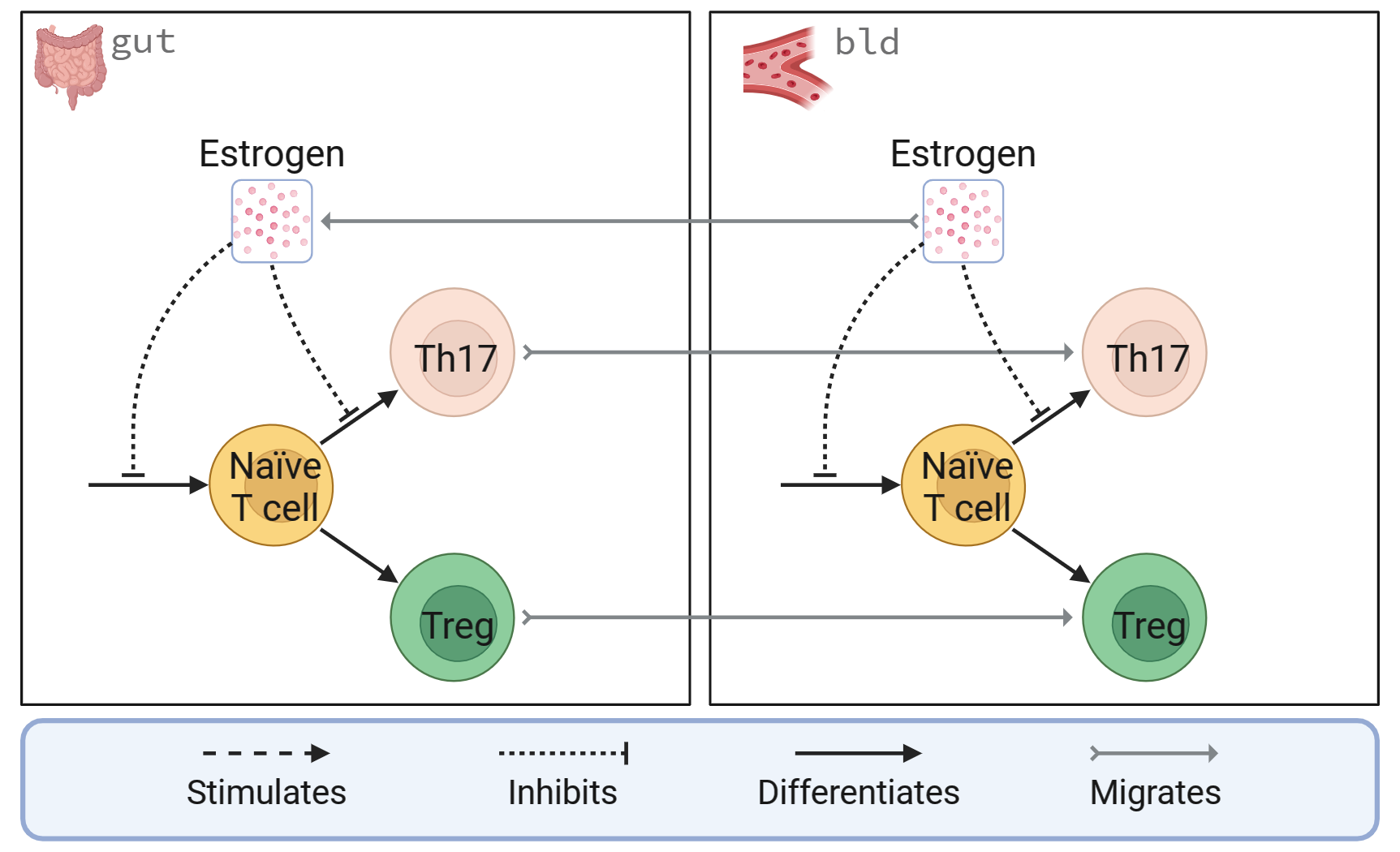
Methods: We developed a two-compartment model of estrogen in the gut and immune system to investigate the mechanisms of estrogen gut-mediated inflammation induced by estrogen-deficient conditions (Figure 1). We first modified our previous butyrate model [7] to include Th17 cells. Our initial model contains ODEs for estrogen, naïve T cells, Tregs, and Th17 cells in gut and blood compartments. We are also exploring an output for and mechanistic features incorporating gut permeability. The model equations consider the processes of species production, differentiation, migration between compartments, degradation, and cell death. We use a mass balance for estrogen and population balances for naïve T cells, Tregs, and Th17 cells. Estrogen interactions with other species are modeled using Hill-type activation and inhibition functions. We estimated degradation and differentiation rates using a mix of normalized in vitro and in vivo data from the literature. Other parameters were calculated using steady-state analysis to fit normalized estrogen levels and T-cell counts observed in healthy female rodents [8, 9].
Results: Our mathematical model will be used to predict the change in T-cell dynamics with changes in endogenous estrogen levels. Our predictions will be compared to in vitro results of Tregs and Th17 cell populations in the gut and blood after OVX. In addition, we will explore the effect of gut permeability variations on T-cell dynamics under healthy and estrogen-deficient conditions. We hypothesize that decreasing gut permeability will increase the ratio of Th17 cells to Tregs under healthy and estrogen-deficient conditions while maintaining a healthy gut barrier during estrogen deficiency will reduce or eliminate gut-mediated inflammation. In the future, we will leverage this model to investigate whether the effect of prebiotic fiber treatment on estrogen-deficient bone loss is significantly affected by gut-mediated inflammation.
Acknowledgments: This work was supported by National Institutes of Health grants R35GM133763, R21AG077640, SUNY Research Seed Grant 231007, and the University at Buffalo. AML is also partially supported by the Arthur A. Schomburg Fellowship Program at the University at Buffalo and the NSF Graduate Research Fellowship Program.
References:
[1] Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012;23(11):576–581.
[2] Tai P, Wang J, Jin H, et al. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2007;214(2):456–464.
[3] Li J-Y, Chassaing B, Tyagi AM, et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 2016;126(6):2049–2063.
[4] Smith BJ, Hatter B, Washburn K, et al. Dried plum’s polyphenolic compounds and carbohydrates contribute to its osteoprotective effects and exhibit prebiotic activity in estrogen deficient C57BL/6 mice. Nutrients. 2022;14(9):1685.
[5] Artoni de Carvalho JA, Magalhães LR, Polastri LM, et al. Prebiotics improve osteoporosis indicators in a preclinical model: systematic review with meta-analysis. Nutr. Rev. 2022;81(8):891–903.
[6] Cook CV, Lighty AM, Smith BJ, Ford Versypt AN. A Review of Mathematical Modeling of Bone Remodeling from a Systems Biology Perspective, Front. Syst. Biol. In press, 2024.
[7] Islam MA, Cook CV, Smith BJ, Ford Versypt AN. Mathematical modeling of the gut–bone axis and implications of butyrate treatment on osteoimmunology. Ind. Eng. Chem. Res. 2021;60(49):17814–17825.
[8] Guo M, Liu H, Yu Y, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. 2023;15(1):2190304.
[9] Qian H, Jia J, Yang Y, Bian Z, Ji Y. A follicle-stimulating hormone exacerbates the progression of periapical inflammation through modulating the cytokine release in periodontal tissue. Inflammation. 2020;43(4):1572–1585.
Figure 1 caption: A two-compartment diagram of estrogen effects on T cells in the gut and blood. Estrogen, Tregs, and Th17 cells migrate from the blood (bld) compartment to the gut (gut) compartment. In both compartments, estrogen inhibits the formation of naïve T cells and their differentiation to Th17 cells.