2023 AIChE Annual Meeting
(2dj) Transport Phenomena and Materials Design in Electrochemical Renewable Energy Storage
Electrochemical energy storage is a promising solution for increasing the utilization of intermittent renewable energy sources like solar and wind. These storage systems allow surplus electric energy to be stored in the form of stable chemical bonds, which can later be converted back to electricity during periods of renewable energy shortage. Given the global scale of energy demands, efficient conversion between electricity and chemicals is crucial. My research interests revolve around comprehending transport phenomena and electron transfer kinetics in electrochemical systems, leveraging my expertise in materials synthesis, nano fabrication, device engineering, operando electrochemistry, spectroscopy, and numerical modeling. I aim to apply this knowledge to a range of electrochemical energy storage systems.
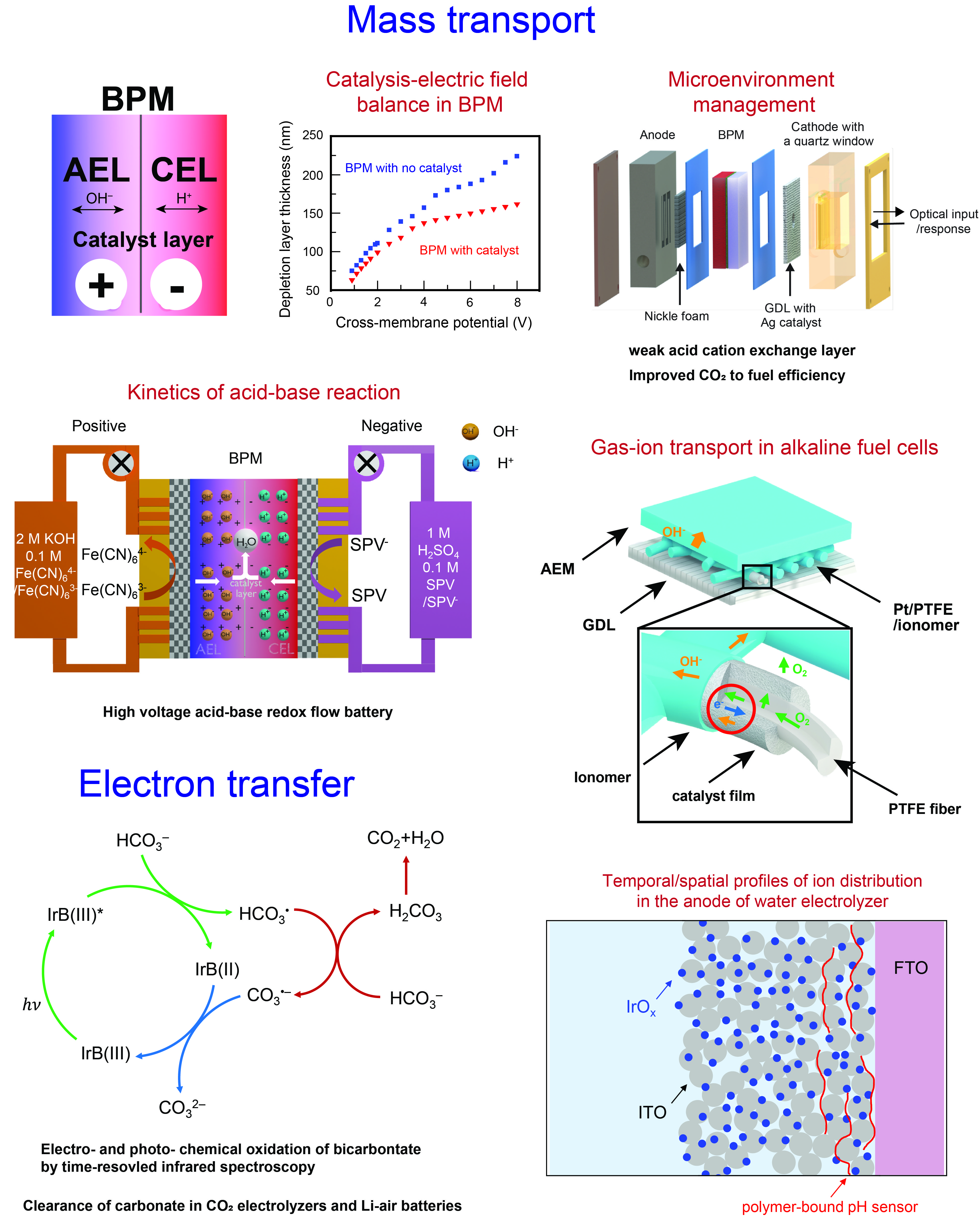
Bipolar membranes (BPMs), composed of a cation exchange layer (CEL) laminated on top of an anion exchange layer (AEL), play a crucial role in maintaining low and high pH conditions for the cathode and anode compartments of a single electrochemical cell. They create an ideal environment for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in water electrolyzers, allowing for the use of earth abundant materials. The main transport problem in BPMs is understanding how the charge carrier transitions from a proton to a hydroxide ion across the CEL/AEL interface. Traditionally, it was believed that this transition is coupled to the water dissociation (WD) reaction, which acts as the source of protons and hydroxide ions within the depletion layer at the CEL/AEL junction. However, the understanding of the mechanisms of WD reactions is still limited within the scientific community. In our first project, we aimed to investigate the correlation between two observed mechanisms that enhance the rate of WD: interfacial catalysis and junction electric field. To achieve this, we constructed BPMs with graphene oxide (GO) as the interfacial catalyst and measured the thickness of the depletion layer using electrochemical impedance spectroscopy (EIS). The two-dimensional nature of GO allowed precise control of catalyst deposition, while the depletion layer thickness served as a convenient metric for the electric field. Our results showed that the presence of an interfacial catalyst decreased the depletion layer thickness, indicating the counterbalanced role of these two mechanisms in promoting WD. These findings were further supported by our numerical modeling results. Based on this new insight, we suggested focusing on improving the performance of WD catalysts rather than solely relying on electric field that saturates at large electric biases. As a result, we designed BPMs with enhanced energy efficiency compared to their commercial counterparts.
Building upon the successful application of BPMs in water electrolyzers, we extended our research to carbon dioxide (CO2) electrolyzers. Electrochemical CO2 reduction requires proton supplies as it is a proton-coupled electron transfer (PCET) process. However, our first-generation BPM-based CO2 electrolyzer exhibited low CO2 to CO conversion efficiency compared to state-of-the-art devices using similar catalysts. This led us to hypothesize that the unique transport properties of BPMs may create a non-ideal cathodic microenvironment for CO2 reduction. To investigate this, we developed new analytical methods to probe the localized microenvironment at the membrane/electrode interface, using electrochemical cells that enabled optical readouts such as microscopic images, UV-Vis absorption, and Raman spectroscopy. We discovered that the low CO2 to CO efficiency was due to the acidic local environment created by BPMs. To address this issue, we coated the BPM surface with weak acid cation exchange layers using layer-by-layer (LBL) assembly. Additionally, we synthesized polymer-bound ratiometric pH sensors that could be easily incorporated into the LBL films. This approach achieved pH measurements at ~10 nm spatial resolution, revealing significantly increased local pH in the LBL-modified BPMs. The LBL coating on the BPM surface resulted in a much-improved conversion efficiency for the CO2 to fuel process.
In the previous two projects, the focus was on studying reverse bias operation mode in BPMs, which has been the dominant research direction in the BPM community. However, in our third project, we shifted our attention to forward bias BPMs due to their technological importance in hydrogen-oxygen fuel cells. Unlike the reverse-biased BPMs where the WD reaction prevails, in forward bias BPMs, the acid-base neutralization (ABN) reaction acts as a sink for proton and hydroxide ions. While the ABN reaction is typically assumed to be diffusion-limited and barrier-less in aqueous solutions, we observed a significant improvement in the reaction rate in forward-biased BPMs with interfacial catalysts, compared to those without catalysts, as indicated by the polarization curves. Our fundamental question was why the kinetics of the diffusion-limited ABN reaction enhanced. Through detailed numerical modeling studies, we discovered that the spatial confinement of protons and hydroxide ions in the CEL and AEL is the reason behind the sluggish ABN reaction. Since ABN requires the presence of both protons and hydroxide ions in the same location, the reaction is limited to the narrow junction region. Building upon this understanding, we developed a BPM-based acid-base redox flow battery (ABRFB) where the positive and negative electrodes were operated at high and low pH, respectively. This setup allowed the potential arising from the pH gradient to be added to the redox potentials of the two electrodes, resulting in a nearly doubled battery potential of 1.6 V compared to the initial 0.9 V. The efficient discharging of the ABRFB relied on the scientific insights regarding the improved rate of the ABN reaction, achieved by equipping the forward biased BPM with interfacial catalysts.
Transport of gas and ionic species in porous electrodes plays a crucial role in determining the performance of fuel cells and water electrolyzers. However, the multi-phase and multi-scale nature of porous electrodes presents challenges for experimental investigations. To address this issue, we employed two strategies: building simpler model systems and developing operando techniques. In the first strategy, we coated the PTFE fibrous support with metallic layers to serve as the catalyst for the oxygen reduction reaction (ORR) on the cathode side of an alkaline fuel cell. We examined the effect of the ionomer layer on the transport of oxygen gas and hydroxide anions and identified an optimal ionomer loading. This novel architecture, featuring the catalyst-coated PTFE fiber layer, demonstrated promising performance in alkaline fuel cells. Leveraging this well-defined architecture, we conducted numerical simulations to elucidate the evolution of an inductive loop in the low-frequency range of the electrochemical impedance spectroscopy (EIS) spectra, thereby relating its origin to the kinetics of the ORR. In the second strategy, we developed operando fluorescence emission spectroscopy to investigate the spatial and temporal distribution of protons within the porous oxygen evolution reaction (OER) electrode. Polymer-bound sensors were localized in the OER electrode, comprising indium-doped tin oxide (ITO)-supported iridium oxide (IrOx) nanoparticles. The high transparency of the IrOx/ITO electrode enabled us to monitor the fluorescence emission spectra of the proton sensor under specific current densities. By obtaining spatial and temporal profiles of proton concentration at various current densities, we discovered that the ITO support acts as a solid-state buffer for the OER reaction. Motivated by this finding, we combined the ITO support with an electrodeposited cobalt OER film. The addition of the ITO support effectively doubled the stable operation time compared to using the pristine catalyst alone.
The formation of carbonate species has long been a challenge in CO2 electrolyzers, alkaline fuel cells, and Li-air batteries. To address this issue in a self-sustained manner, we propose direct electrochemical oxidation of carbonate at the anode. Through infrared spectro-electrochemistry and time-resolved infrared spectroscopy, we have elucidated the detailed mechanisms of bicarbonate oxidation in non-aqueous environments. Our findings reveal that the rapid first electron transfer from bicarbonate generates the acidic bicarbonate radical. This radical then reacts with the bicarbonate anion to produce carbonic acid, which subsequently decomposes into CO2 and H2O. Furthermore, we have prepared and extensively characterized peroxybicarbonate, previously hypothesized as a two-electron oxidation intermediate of bicarbonate. Our investigations demonstrate that peroxybicarbonate undergoes an additional two-electron oxidation process, resulting in the production of CO2 and oxygen gas. These discoveries offer a promising approach for effectively clearing carbonate species in various technologically important electrochemical devices.
Teaching Interests
Throughout my education and research career, I have acquired extensive experience in teaching a diverse range of topics to students. Drawing upon these experiences, I am keen on instructing both undergraduate and graduate level courses in general chemistry, materials chemistry, mathematical method, and materials balance. Additionally, I am enthusiastic about offering an electrochemistry course that delves into the microscopic scale description of the electrode/electrolyte interface. This course will encompass the distribution of ions and substrates in solution and how the presence of an electrode influences this distribution. Students will be introduced to classical models of binding isotherms, charge transfer, as well as novel electrochemical and operando techniques. Practical laboratory sessions will provide students with hands-on experience in learning and interpreting popular electrochemical techniques. Furthermore, I envision teaching courses that focus on renewable energy storage and provide an overview of important transport-related topics in electrochemical systems. These courses will explore the fundamental principles and applications of electrochemical energy storage, including batteries and fuel cells, and address the various transport phenomena involved.