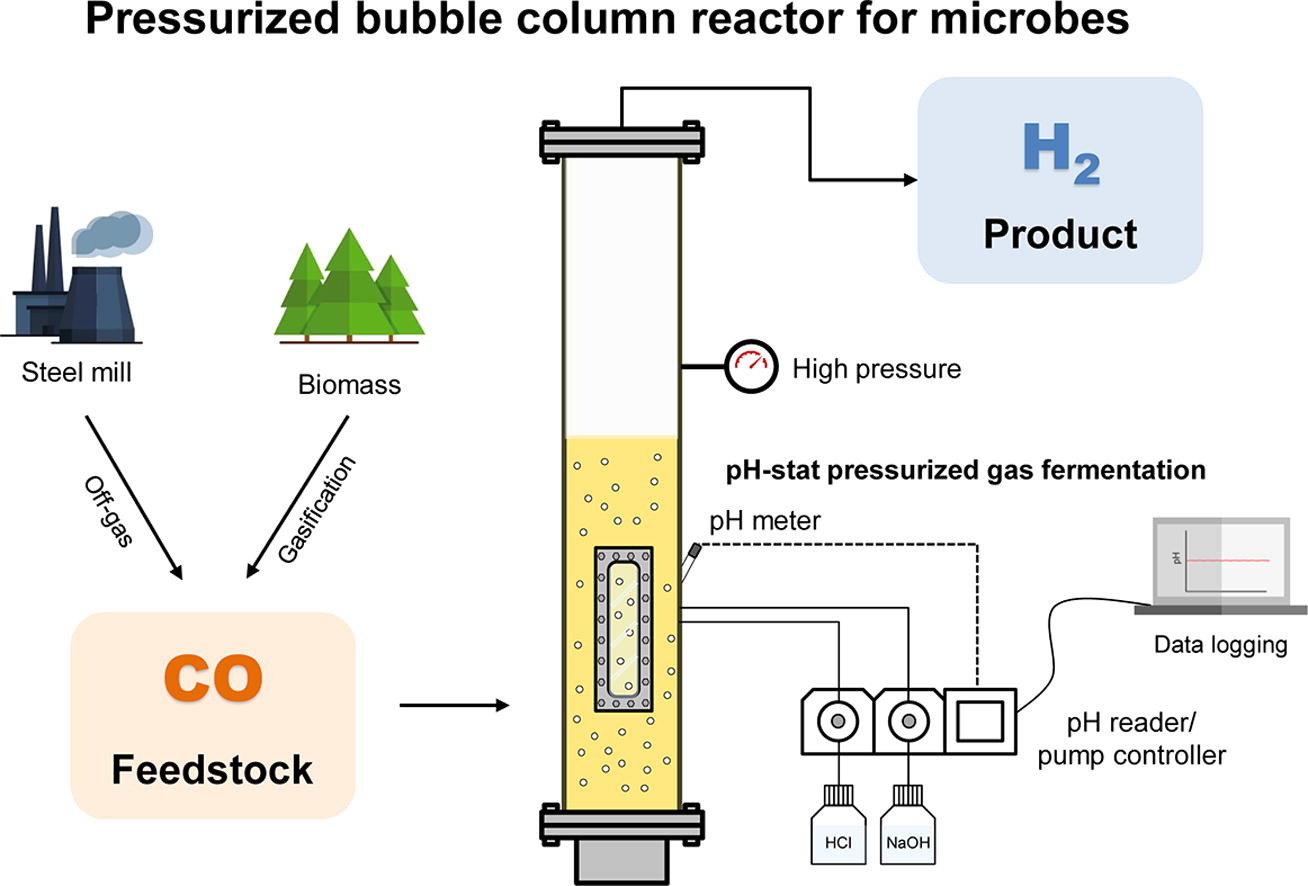
Gas fermentation is an environmentally sustainable approach that converts syngas derived from biomass or waste into valuable products. In this study, a bubble column reactor was designed to pressurize the system and enhance the mass transfer rate between gas and liquid while reducing the energy consumption by medium agitation. The hydrogenic CO-oxidizer, Thermococcus onnurineus, was cultured under ambient pressure with an initial inlet gas composition of 60% CO and 40% N
2. The maximum H
2 productivity of 363 mmol/l/h was achieved without pH adjustment. However, when additional pressure was applied, the pH of the system rapidly declined, which may be attributed to the increased solubility of CO
2 under pressure. By controlling the pH, H
2 productivity increased up to 450 mmol/l/h, which is comparable to the H
2 productivity reported in a continuous stirred tank reactor. These results indicate the energy-saving potential of bubble column reactors in gas fermentation. Moreover, this finding may be applicable to other gas fermentation processes, as syngas itself contains CO
2, and many microbial processes release CO
2.