2020 Virtual AIChE Annual Meeting
(377d) Digital Design of End-to-End Manufacturing Process for Mefenamic Acid Using Mechanistic Modeling
Authors
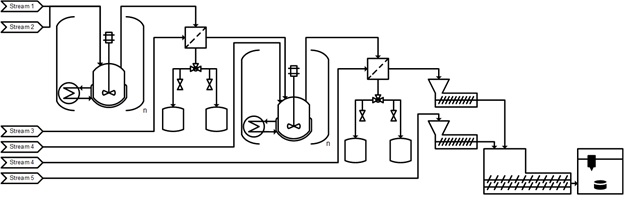
This work will outline the quantitative analysis performed on the manufacturing process for mefenamic acid by building the end-to-end mechanistic flowsheet model Each element of the drug substance production step, including synthesis step, crystallization and separation are validated using process data individually. Once each unit operation step in the production step is validated, a systems model was configured to provide a quantitative representation of the end-to-end production process including drug substance, drug product and product performance elements. This end-to-end model was subsequently utilized to develop a quantitative understanding of the effect of process disturbances, raw material variability, process parameters, formulation parameters and model uncertainty on CQAs (e.g. tablet properties) and manufacturability. Along with the analysis, the relative influence of those factors (sensitivity indices) on the desired CQAs will also be presented. Furthermore, the impact of batch and continuous operational elements of the process will also be probed. This work helps to identify any existing gaps in the scientific understanding of the effects of some of the API attributes on the desired KPIs during this end-to-end flowsheet analysis will also be discussed.