2025 AIChE Annual Meeting
(287d) In Vitro Tissue Models of Neural Cell Development Towards Myelination and Remyelination
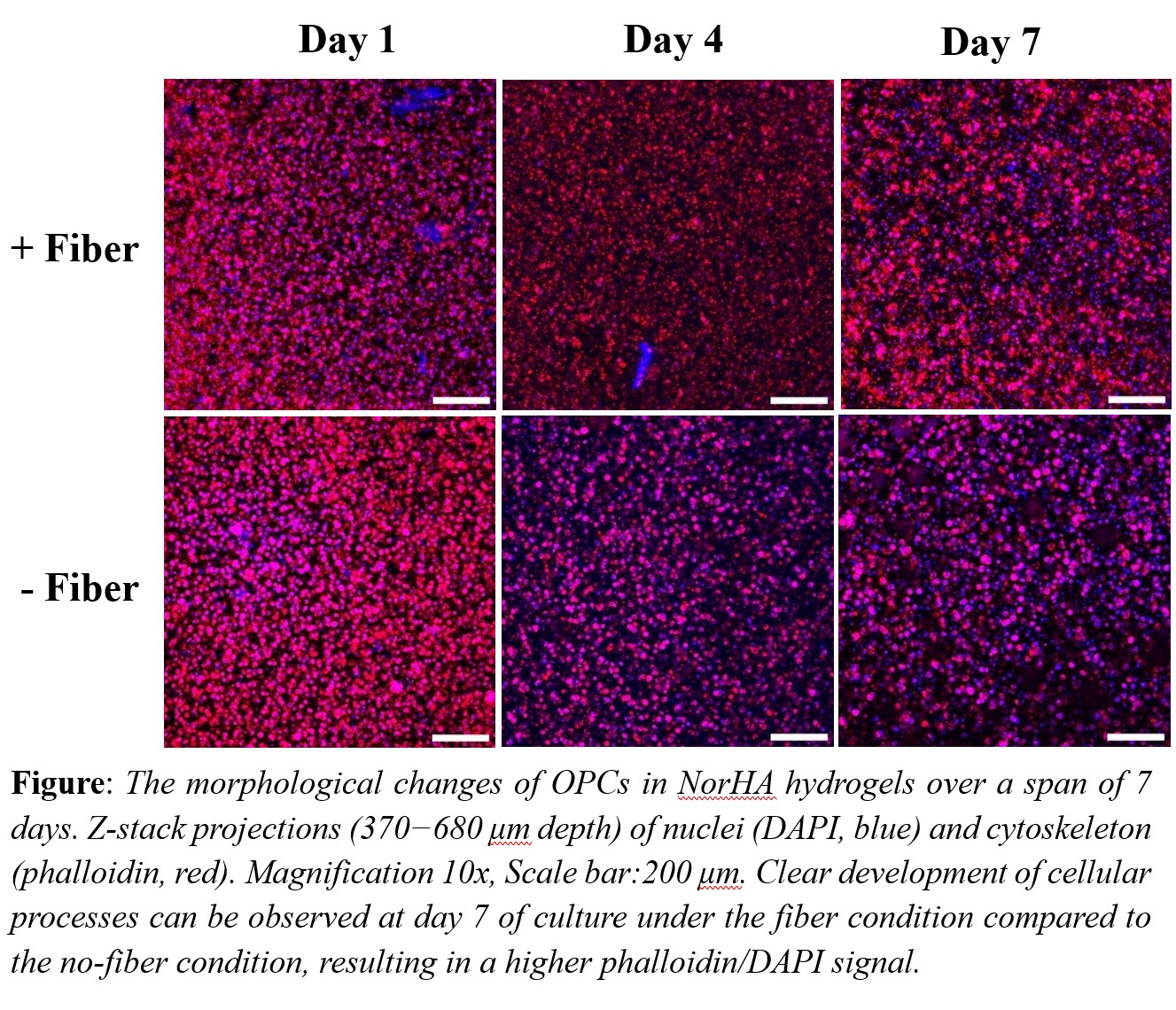
Methodology: Hyaluronic acid (HA) was modified with norbornene groups to enable a light-mediated thiol-ene reaction between the norbornene groups and dithiothreitol (DTT), resulting in hydrogel formation. The fibers, consisting of 2 wt% methacrylate-functionalized hyaluronic acid (MeHA), 3 wt% polyethylene oxide (PEO). and 0.05 wt% Irgacure 2959 photoinitiator were spun using a Spraybase electro spinner, and the dry fibers were crosslinked under 254 nm UV light. Following crosslinking, the fibers were resuspended in phosphate-buffered saline (PBS) for at least 48 hours. The fibers were then fragmented into smaller pieces by passing them through an 18-gauge needle followed by a 21-gauge needle before incorporating them into the gel solution. OPCs (5 x 106 cells/mL) and fibers (1 wt%) were co-encapsulated in 1.5 wt% norbornene functionalized hyaluronic acid (NorHA) hydrogels by crosslinking at 4 mW/cm2 of UV light for two minutes. Gels were designed to match the storage modulus of brain tissue (200–2000 Pa) by incorporating DTT at a 31.5% crosslinking efficiency. To assess cell morphology over the course of 7 days, gels were stained with DAPI and phalloidin to label nuclei and F-actin, respectively, and imaged via confocal microscope.
Results: Rheology analysis showed that a storage modulus of approximately 700 Pa was achieved with 1.5 wt% NorHA. Additionally, bulk rheology analysis revealed no significant differences in storage modulus between hydrogels with and without fibers. The dry fiber diameter was quantified as 410 ± 8 nm, while the swollen fiber diameter was measured at 2147 ± 220 nm. Already on day 1 OPCs exhibit a clear morphological change when there are fibers present (see Figure). By day 7, OPCs extend clear processes in hydrogels with fibers, whereas OPCs encapsulated without fibers show no developed processes on day 7, and their spherical shape remains unchanged over time. DAPI and Phalloidin signal areas were quantified, and the area of phalloidin/DAPI signal shows a fivefold increase in the images with the fiber condition at day 7 compared to the no-fiber condition. Furthermore, live/dead analysis indicated no significant difference in the number of live cells between the two conditions at any time point.
Conclusion: Both hydrogel-only and fiber-containing samples permitted OPC proliferation over time, with no significant difference in on cell viability. This confirms that both NorHA hydrogels and MeHA fibers are promising candidates for in vitro models in our research. The higher phalloidin/DAPI signal area observed in fiber-containing samples suggests that the presence of fibers, which mimic neuronal axons, supports OPC maturation by promoting process extension. Given the observed differences in cell morphology, we will further investigate how fiber properties such as diameter and stiffness affect OPC behavior. Additionally, OPC differentiation will be assessed via gene profiling using Nanostring technology to identify dysregulated genes. These future studies will enhance our understanding of how fiber properties influence cell behavior and help guide the development of biomaterials for improved neural regeneration.
References
1.Thurman, D.J., Alverson, C., Dunn, K.A., Guerrero, J. and Sniezek, J.E., 1999. Traumatic brain injury in the United States: a public health perspective. The Journal of head trauma rehabilitation, 14(6), pp.602-615.
2.Trapp, B. D.; Peterson, J.; Ransohoff, R. M.; Rudick, R.; Mörk, S.; Bö, L. Axonal Transection in the Lesions of Multiple Sclerosis. N Engl J Med 1998, 338 (5), 278–285. https://doi.org/10.1056/NEJM199801293380502.
3.Long, K. L. P.; Breton, J. M.; Barraza, M. K.; Perloff, O. S.; Kaufer, D. Hormonal Regulation of Oligodendrogenesis I: Effects across the Lifespan. Biomolecules 2021, 11 (2), 283. https://doi.org/10.3390/biom11020283.
4.Puhl, D. L.; Funnell, J. L.; Nelson, D. W.; Gottipati, M. K.; Gilbert, R. J. Electrospun Fiber Scaffolds for Engineering Glial Cell Behavior to Promote Neural Regeneration. Bioengineering 2020, 8 (1), 4. https://doi.org/10.3390/bioengineering8010004.
5.Liu, F.; Xu, J.; Wu, L.; Zheng, T.; Han, Q.; Liang, Y.; Zhang, L.; Li, G.; Yang, Y. The Influence of the Surface Topographical Cues of Biomaterials on Nerve Cells in Peripheral Nerve Regeneration: A Review. Stem Cells International 2021, 2021, 1–13. https://doi.org/10.1155/2021/8124444.