2025 AIChE Annual Meeting
(607g) In Vitro Evaluation of the Antiviral Activity of the RD10 Peptide Selected By Artificial Intelligence
Authors
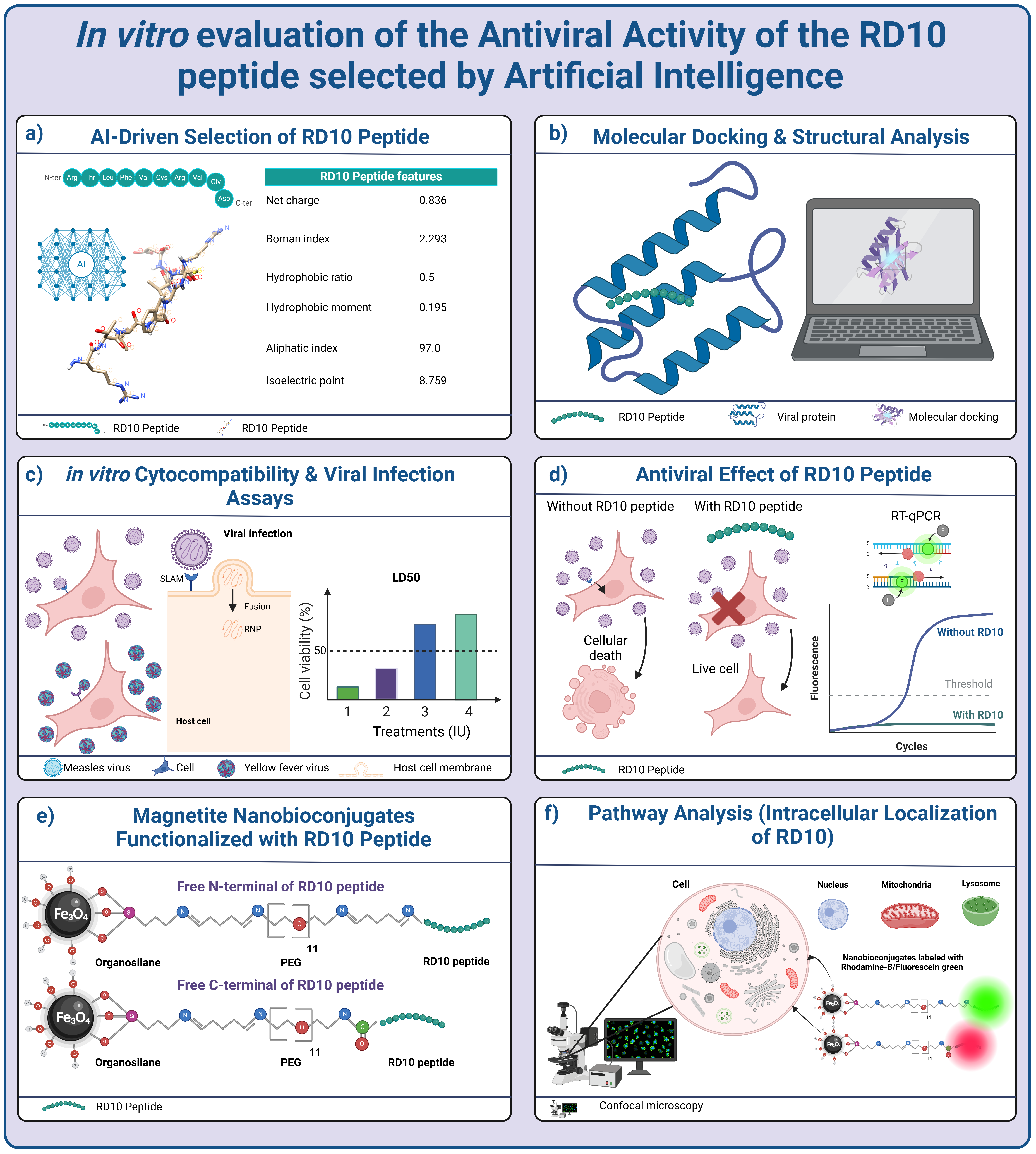
Additionally, we investigated the intracellular localization of RD10 using magnetite nanobioconjugates functionalized with RD10 peptide and labeled with either Rhodamine B or fluorescein green. The subcellular distribution was analyzed in mitochondria, lysosomes, and nucleus. We also compared the antiviral effects of RD10 in its free form and when functionalized with magnetite nanoparticles (MNPs). In these conjugates, RD10 was functionalized either via its free N-terminal (MNP-RD10 (C-terminal free)) or its free C-terminal (MNP-RD10 (N-terminal free)). Our results demonstrated that both free and functionalized RD10 reduced viral replication and improved cell viability in infected cultures. However, RD10 conjugated to MNPs exhibited a stronger protective effect, particularly at higher viral loads (10 LD₅₀ and LD₅₀) in both MMR and YF infections. Notably, the functionalization strategy influenced antiviral efficacy: MNP-RD10 (C-terminal free) showed superior protective effects at 10 LD₅₀, whereas MNP-RD10 (N-terminal free) was slightly more effective at lower viral loads. These findings suggest that RD10 orientation on the nanoparticle surface plays a key role in its bioactivity, potentially affecting its interaction with viral components or cellular targets. To further explore its mechanism of action, we performed molecular docking simulations to predict RD10’s interactions with key viral proteins involved in host cell membrane binding. Specifically, we analyzed the hemagglutinin-neuraminidase (HN) protein of the mumps virus, the hemagglutinin (HA) protein of the measles virus, the glycoprotein E1 of the rubella virus, and the envelope protein (E) of the yellow fever virus. These simulations provided insights into potential binding sites and interaction affinities, supporting the experimental findings on RD10’s antiviral effects.
These results highlight the enhanced antiviral potential of RD10-functionalized magnetite conjugates and emphasize the importance of peptide orientation in optimizing antiviral efficacy. The integration of AI-driven peptide selection, in vitro evaluations, molecular docking simulations, and cell viability assessments underscores RD10’s potential as a novel antiviral agent. This research contributes to the development of innovative antiviral therapies.