2025 AIChE Annual Meeting
(413e) A Vanadium Redox Flow Process for Electrochemical Carbon Capture and Energy Storage: Proof-of-Concept Demonstration and Techno-Economic Analysis
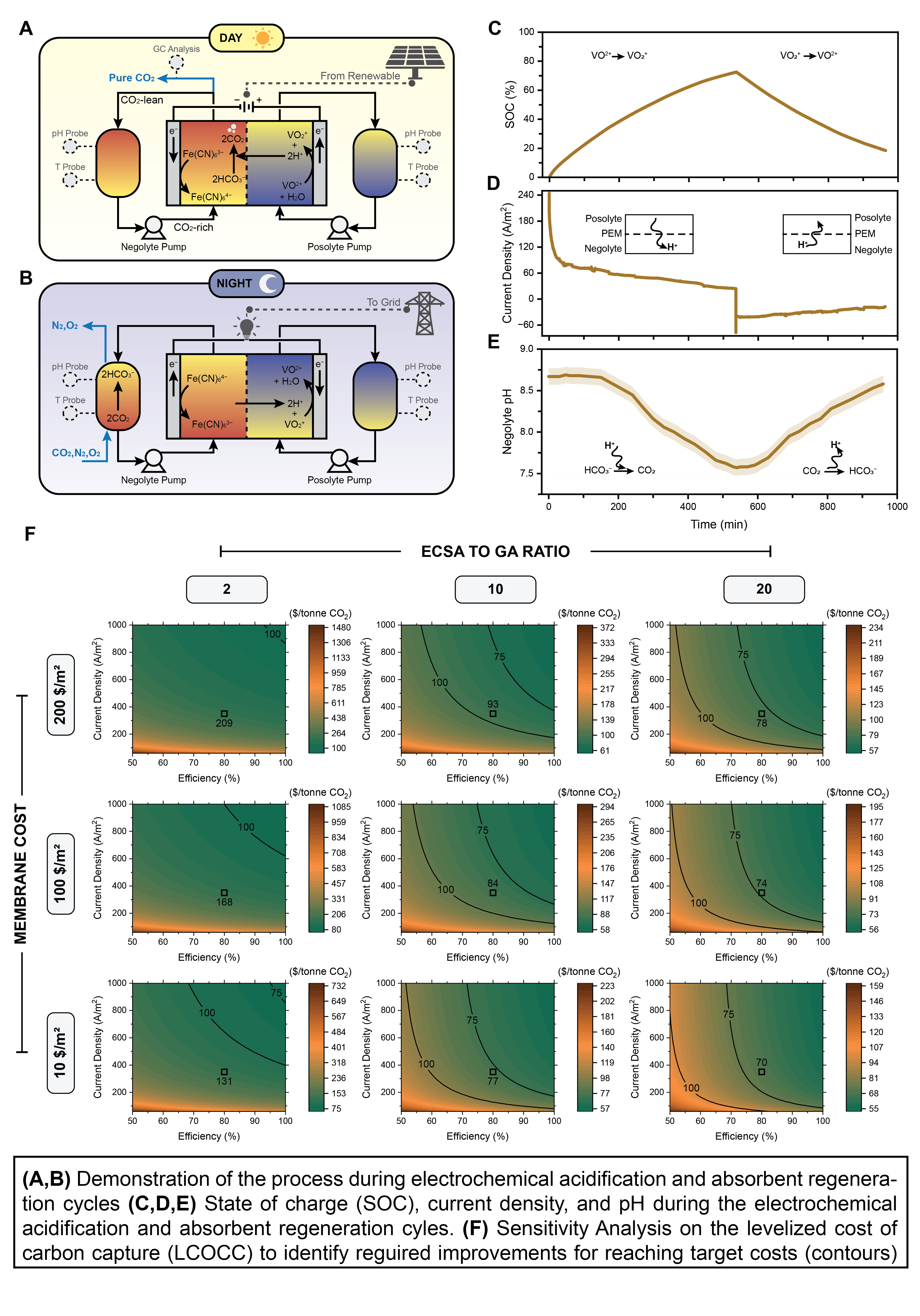
Electrochemical impedance spectroscopy (EIS) was used to study the redox kinetics, highlighting significant performance improvements achieved through plasma treatment of graphite electrode surfaces. This treatment decreased the contact angle, increased hydrophilicity, and improved wettability, thereby enhancing electrochemical surface activity. Consequently, a 43% reduction in charge transfer resistance was achieved, directly correlating to enhanced current density and faster reaction kinetics. Additionally, comprehensive mass transfer studies established an optimized electrolyte composition consisting of a balanced 1:1 ratio of redox-active species to background electrolyte due to the trade-off between electrical conductivity and Faradaic efficiency. Bench-scale experiments demonstrated reversible system operation, achieving an energy consumption of approximately 54 kJ/mol CO2—comparable or superior to existing electrochemical capture methods reported in the literature. Additionally, the reversibility of the developed process was studied by its operation over multiple charge and discharge cycles.
A detailed thermodynamic modeling effort was also carried out to support experimental observations and serve as the backbone of techno-economic analysis (TEA). The process modeling included four stages: 1-CO2 absorption using potassium carbonate (H2CO3) in the absorber, 2-electrochemical acidification during the charge cycle, 3-CO2 outgassing in the flash tank, and 4-electrochemical absorbent regeneration during the discharge cycle. Equilibrium speciation analysis of dissolved inorganic carbon (DIC) species, including bicarbonate (HCO3−), carbonate (CO32−), and carbonic acid (H2CO3), was studied because of proton concentration modulation on the carbon capture process at different states of charge (SOC). The model revealed a substantial increase in CO2 partial pressure during proton-driven acidification, confirming the strong driving force for gas-phase CO2 desorption upon pH reduction. Furthermore, modeling outcomes guided the optimization of electrolyte concentrations and identified critical operational parameters influencing overall system performance, such as state of charge (SOC), cell potential and and redox speciation control within electrolytes.
Complementing the experimental proof-of-concept studies, an extensive techno-economic analysis (TEA) was performed to evaluate the economic feasibility and potential commercial scalability of the technology. The TEA utilized detailed cost and performance metrics from commercially available VRFB literature, addressing a common limitation in ECC research due to the low technology readiness levels (TRL) and associated scarcity of accurate scaled-up cost data. This approach allowed for a more realistic TEA and reducing uncertainties typically associated with early-stage electrochemical systems. The economic analysis identified two primary capital expenditure drivers: (i) the electrode active surface area (ECSA)-to-geometric area (GA) ratio, critical for enabling three-dimensional scaling of electrochemical modules using porous electrodes, and (ii) membrane costs, particularly for proton exchange membranes (PEMs) such as Nafion. Meanwhile, operating expenses were mainly influenced by cell potential and Faradaic efficiency. A systematic sensitivity analysis conducted within the TEA framework evaluated the individual and combined impacts of these parameters, specifically examining the ECSA-to-GA ratio, membrane prices, current density, cell potential, and Faradaic efficiency. Additionally, the study introduced a novel "cost-target analysis," designed to assess the synergistic effects of simultaneous improvements across multiple performance metrics rather than evaluating them separately. This multi-variable approach identified operational regimes capable of achieving targeted capture costs of $100 and $75 per tonne of CO2 through incremental improvements in key parameters. Specifically, the analysis showed that reducing Nafion membrane prices to below $200/m2—or alternatively, adopting cost-effective membranes such as sulfonated poly(ether ether ketone) (SPEEK)—is essential for meeting these economic targets. However, the performance and long-term durability of SPEEK membranes must be improved to ensure viability at scale. Additionally, increasing the ECSA-to-GA ratio above 10 was identified as another critical requirement. These findings provide clear guidelines for future research and development efforts aimed at improving the cost-competitiveness of ECC technologies.
References
1. Afshari M, Refaie A, Aleta P, Hassan A, Rahimi M. A Vanadium Redox Flow Process for Carbon Capture and Energy Storage. ACS EST Eng. [Internet] 2025 [cited 2025 Jan 30];Available from: https://doi.org/10.1021/acsestengg.4c00631