2025 AIChE Annual Meeting
(390ae) Valorization of Plastic Waste into Fuels/Chemicals: Process Development and Integrative Analyses
Mechanical recycling is widely employed; however, it is energy-intensive and tends to produce low-quality recycled materials due to degradation of properties upon repeated processing. Consequently, its long-term feasibility within a circular economy system is limited (Sánchez-Rivera & Huber, 2020).
In contrast, chemical recycling methods such as catalytic pyrolysis and hydrogenolysis have gained attention as alternatives that enable the conversion of polyolefins into high-value fuels and chemical feedstocks, thereby facilitating true resource circulation and mitigating environmental impact (Zou et al., 2023; Chu et al., 2023). In particular, hydrogenolysis using Ru-based catalysts is regarded as a technology capable of achieving both economic and operational efficiency, as it enables the production of liquid fuels with high selectivity while suppressing methane formation (Rorrer et al., 2020; Peczak et al., 2023).
However, such chemical processes often require high-temperature and high-pressure conditions, resulting in significant energy consumption, carbon emissions, and high capital costs (Lee et al., 2024). These factors pose substantial challenges for industrial-scale deployment of technology.
To address these issues, this study proposes a hydrogen-substitute-based hydrogenolysis process. This process is expected to be a promising technology that can convert polyolefin waste plastics into fuels such as gasoline and LPG, as well as petrochemical feedstocks like toluene and xylene, under milder reaction conditions, while achieving both energy efficiency and environmental sustainability (Frączak, 2021; Seifali Abbas-Abadi et al., 2023).
This paper aims to evaluate the technical and environmental feasibility of this innovative recycling process and assess its potential to resolve the economic and ecological challenges associated with polyolefin-based plastic waste.
The core of this process is the utilization of decalin, a type of liquid organic hydrogen carrier (LOHC), which can reversibly store and release hydrogen through a dehydrogenation reaction. When the dehydrogenation of decalin is coupled with the hydrogenolysis of polyolefins, hydrogen can be consumed in situ, allowing for polymer chain cleavage without external hydrogen supply or separation units. Compared to conventional pyrolysis or hydrogenolysis, this integrated approach suppresses gaseous byproduct formation and enables effective depolymerization under milder temperature and pressure conditions.
External hydrogen typically produced from steam methane reforming or water electrolysis depends heavily on centralized supply infrastructure and incurs high transport costs. The LOHC strategy reduces transportation burdens; however, it necessitates a decalin dehydrogenation step upstream. To assess the industrial feasibility of the proposed technology, experimental results were scaled up and modeled for a system designed to process 240 tons of low-density polyethylene (LDPE) waste per day (10 tons per hour). This scale was intentionally selected to reflect a future commercially viable scenario (Yadav, G. et al.). The process flow diagram integrates plastic depolymerization, separation of liquid and gaseous fuels, heat and power generation, storage, and utilities. Process simulations were performed using Aspen Plus V12. Capital cost estimations for major units such as distillation columns, pumps, and heat exchangers were conducted using Aspen Economic Analyzer, while cost data for reactors and filters were scaled using National Renewable Energy Laboratory (NREL) benchmarks. All capital and operating costs were normalized using the Chemical Engineering Plant Cost Index (CEPCI).
The reactor system of this process is based on laboratory-scale experimental data using 0.5 wt% Pt/HZSM-5 (Si/Al = 30) catalyst. To characterize catalyst performance, hydrogen release profile, and product selectivity, experimental validation was conducted in a continuous flow reactor. The Pt/HZSM-5 catalyst exhibited high activity and stability under moderate conditions (~330°C), achieving concurrent hydrogen release and polyolefin cleavage due to its bifunctional catalytic properties. The resulting hydrocarbon mixture primarily consisted of C5–C16 linear and branched alkanes and aromatic compounds, with minimal coke formation and negligible gas generation. The most effective experimental case demonstrating decalin dehydrogenation and polyolefin hydrogenolysis was selected and scaled up for process simulation.
Three process configurations were evaluated: Case 1 is the tandem process where decalin dehydrogenation and polyolefin hydrogenolysis occur in a single reactor, Case 2 is the two-stage sequential process with decalin dehydrogenation followed by hydrogenolysis using the released hydrogen, and Case 3 is the direct hydrogenolysis process using externally supplied hydrogen. For configurations Case 2 and Case 3, the hydrogenation data were based on experiments conducted under hydrogen-rich conditions, and it was assumed that hydrogen feed was three times the stoichiometric consumption to maintain similar reactivity. Accordingly, decalin flow rates in configuration (2) were adjusted to match hydrogen demand.
In the base case(Case 1), a combination of distillation and extractive separation was employed for fuel product recovery. Integration of sulfolane-based extraction enabled efficient recovery of aromatic hydrocarbons (BTX), and solvent recycling minimized waste generation and operational costs. All three configurations employed unreacted LDPE separation and recovery units. A portion of residual low-value C1–C2 hydrocarbons was combusted to meet heat and power demands. A pinch analysis was conducted to design a heat exchanger network that maximized energy recovery. Thermal integration reduced heating utility demand by 59% and cooling demand by 60%, thereby decreasing operational costs and reducing fuel-derived emissions and cooling water usage. Similar energy savings were achieved in the comparative cases(Case 2 and Case 3): 50% and 49% reductions for Case 1, and 56% and 47% for Case 2, in heating and cooling requirements, respectively.
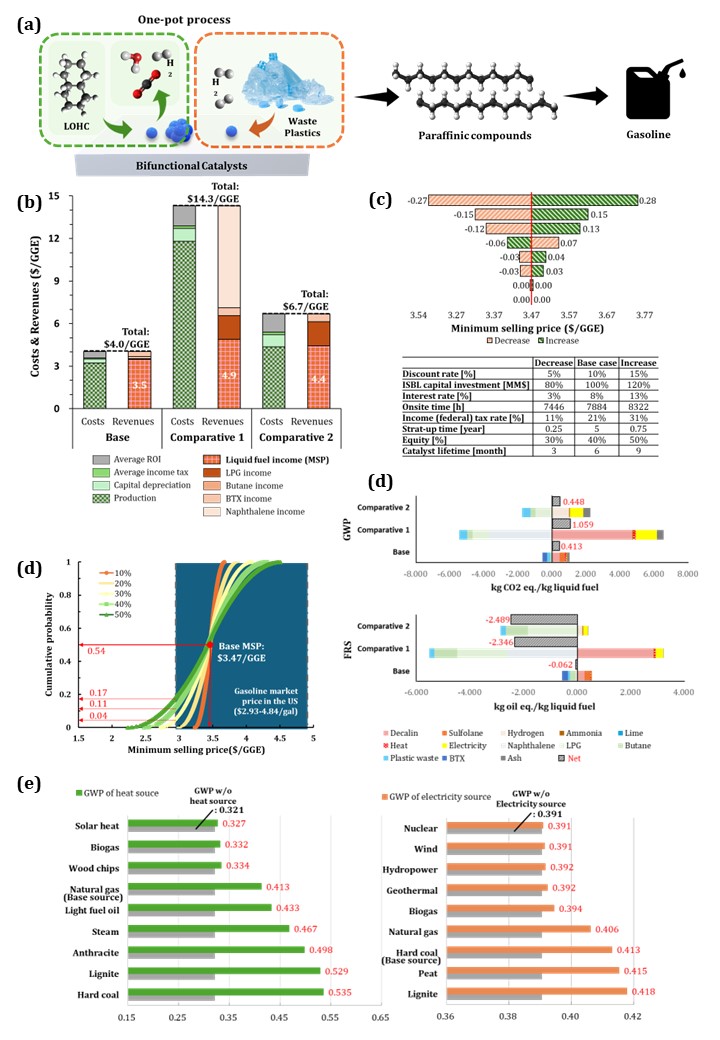
Among the three configurations, the base case achieved the highest target product yield, generating approximately 7.2 tons of gasoline per hour, corresponding to a 65.4% carbon efficiency relative to the total carbon in plastic and decalin feed. Additionally, LPG and butane were recovered as byproducts with carbon efficiencies of 3.8% and 9.7%, respectively, while BTX was obtained with 10.8% carbon efficiency. Although comparative Case 1 enabled additional production of naphthalene, it consumed significantly more decalin (4.6 tons/hour). While the external hydrogen supply route Case 2 was structurally simpler, both comparative cases exhibited lower gasoline yields (20.8% and 30.7% carbon efficiency, respectively) and higher minimum selling prices (MSPs) than the base case. Moreover, increased energy demand due to compressor use raised environmental burdens even after energy integration.
Techno-economic analysis (TEA) was conducted using a discounted cash flow method, assuming a 10% internal rate of return (IRR), 30-year plant lifetime, and a 3-year capital recovery period. The MSP of gasoline in the base case was estimated to be $3.5 per gasoline gallon equivalent (GGE). Sensitivity analysis indicated that the discount rate was the primary driver of MSP; a ±5% variation in discount rate led to an 8% change in MSP, ranging from $3.2 to $3.75/GGE. Monte Carlo simulations incorporating six key economic drivers—such as decalin replenishment rate, LDPE feedstock cost, and byproduct market prices—demonstrated that MSP remained within a competitive market range under realistic economic fluctuations, suggesting resilience to supply chain volatility.
To assess environmental sustainability, a life cycle assessment (LCA) was conducted in accordance with ISO 14040 and 14044 standards, using a gate-to-gate system boundary and 1 kg of gasoline-equivalent product as the functional unit. The ReCiPe 2016 midpoint method in SimaPro 9.1, using the Ecoinvent 3.6 database, was employed to quantify global warming potential (GWP) and fossil resource scarcity (FRS). The base case yielded a GWP of 0.413 kg CO₂ eq./kg and an FRS of –0.062 kg oil eq./kg. Case 2 and 3 exhibited higher GWP values (1.059 and 0.448 kg CO₂ eq./kg, respectively), primarily due to energy-intensive decalin regeneration and hydrogen compression.
A sensitivity analysis of energy sources revealed that replacing natural gas and coal with renewable sources such as solar or biogas could reduce GWP by up to 21%. Electricity supplied by nuclear or wind sources showed a 5% GWP reduction compared to the baseline, while lignite-based electricity slightly worsened emissions. These results underscore the critical role of utility source selection in minimizing environmental impacts.
Future research will focus on identifying alternative LOHCs with lower environmental burdens and evaluating long-term catalyst deactivation, sintering, and regeneration behavior to ensure commercial-scale process viability. In summary, while each process configuration presents trade-offs, the base case demonstrates superior performance in terms of carbon efficiency, environmental sustainability, and economic feasibility. The proposed process offers a promising route for low-emission, cost-effective gasoline production while eliminating infrastructure burdens associated with hydrogen supply.
References:
[1]. Tan, Y., et al. Catalytic Chemical Recycling and Upcycling of Polyolefin Plastics. Giant, 2024.
[2]. Rorrer, J. E., et al. Conversion of Polyolefin Waste to Liquid Alkanes with Ru-Based Catalysts under Mild Conditions. JACS Au, vol. 1, 2021, 8–12.
[3]. Sánchez-Rivera, K. L., and G. Huber. Catalytic Hydrogenolysis of Polyolefins into Alkanes. ACS Central Science, vol. 7, 2020, 17–19.
[4]. Zou, L., et al. Chemical Recycling of Polyolefins: A Closed-Loop Cycle of Waste to Olefins. National Science Review, vol. 10, 2023.
[5]. Chu, M., et al. Site-Selective Polyolefin Hydrogenolysis on Atomic Ru for Methanation Suppression and Liquid Fuel Production. Research, 2023.
[6]. Peczak, I. L., et al. Treasuring Trash: Pt/SrTiO₃ Catalysts Process Plastic Waste into High-Value Materials. Matter, 2023.
[7]. Lee, S., et al. Sustainable Chemical Recycling of Waste Plastics into Olefins through Low-Pressure Hydrothermal Liquefaction and Microwave Pyrolysis: Techno-Economic Analysis and Life Cycle Assessment. Energy Conversion and Management, 2024.
[8]. Frączak, D. Chemical Recycling of Polyolefins (PE, PP): Modern Technologies and Products. Current Topics in Recycling, IntechOpen, 2021.
[9]. Seifali Abbas-Abadi, M., et al. Challenges and Opportunities of Light Olefin Production via Thermal and Catalytic Pyrolysis of End-of-Life Polyolefins: Towards Full Recyclability. Progress in Energy and Combustion Science, vol. 95, 2023, 101046.
[10]. Yadav, Geetanjali, et al. Techno-Economic Analysis and Life Cycle Assessment for Catalytic Fast Pyrolysis of Mixed Plastic Waste. Energy & Environmental Science, vol. 16, 2023, 3638–3653.