2025 AIChE Annual Meeting
Utilizing Lab-on-a-Chip Technology to Leverage Imaging Cytometry for Immune Cell Analysis
To deliver decentralized QC and enable cell therapy manufacturing in a wider range of settings, lab-on-a-chip technologies have proven to be low-cost alternatives that forgo the need for large operating spaces, skilled operators, and labor-intensive sample preparation practices such as labeling. Such technologies, typically following a portable biochip form factor, often rely on immunoaffinity to assess cell health using antibody-antigen interactions in compartments of the device. Previous research has developed biochips for apoptotic cell detection, immune cell analysis, and single-cell isolation— all based on surface markers. We aim to expand this platform to examine intracellular markers, providing a more comprehensive analysis of cellular health in an integrated biochip format, and thus accomplish a level of analytical insight similar to that of traditional flow cytometers. This design can also provide cost-effective and time-efficient sample testing for point-of-care diagnostics.
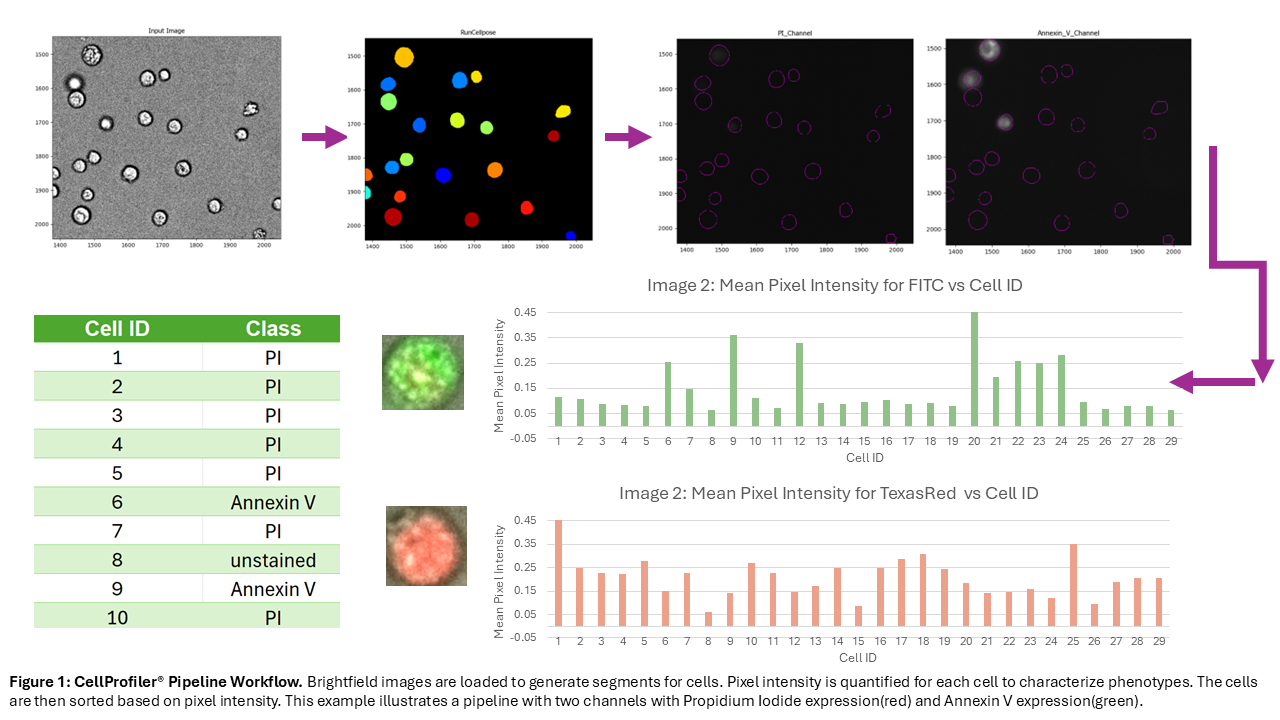
Herein, we propose the development of an optical biochip and an accompanying image processing pipeline for phenotypic and morphological assessments. The biochip is made via soft lithography, casting the fluidic features in polydimethylsiloxane (PDMS) and sealing them with a standard laboratory glass slide through an oxygen plasma-activated bonding procedure. The chambers patterned in the PDMS allow the cells to remain static before undergoing analysis. Cells and fluorescent dyes are loaded into the biochip for on-chip labelling before brightfield and appropriate fluorescent channel images are taken. These images are then passed into our image processing pipeline built in CellProfiler®—a free, open-source software—that performs cell segmentation and declustering using a pre-trained deep learning model (Cellpose), then uses these segments to quantify expression level in each cell for all fluorescent channels recorded. These measurements are then aggregated to report the population phenotypic profile. The resulting output is a spreadsheet with the cell ID and fluorescent expression in each channel, allowing for traceable, single cell analysis. We aim to benchmark this work against flow cytometry in future experiments to assess its accuracy and its potential as cost effective QC solution for cell manufacturing.
- “Pficell - Profound Future Focused Innovative Cell Therapy.” FDA-Approved Cell Therapies for Cancer, 2025, pficell.ca/en/news-cancer-fda.
- “Flow Cytometry Cost: Cost Comparison of Separation Methods.” Akadeum Life Sciences, 18 May 2025, www.akadeum.com/technology/cost-comparison-of-cell-separation-methods/#….