2025 AIChE Annual Meeting
(570b) Upgrading Waste Biomass Via an Enantioselective Chemo-Enzymatic Reaction: Opportunities in Process Strategy
Authors
The chemo-enzymatic reaction is considered a promising technology due to its high selectivity and environmental benefits. However, chemical catalysts usually require organic solvent conditions, whereas enzymes function in aqueous conditions. Combining these two catalysts with different reaction conditions has been considered difficult (Rudroff et al., 2018; Gröger et al., 2022). Recently, Cha et al. (in press) integrated their developed enzymes with an acid-catalyzed reaction in an aqueous phase as a chemical catalytic reaction. This approach minimized differences in reaction conditions, resulting in an economically and environmentally feasible process that produced optically pure (R)-GVL with an enantiomeric excess of over 99%. Nevertheless, despite unified solvent conditions, significant differences remain between chemical and biological catalytic environments.
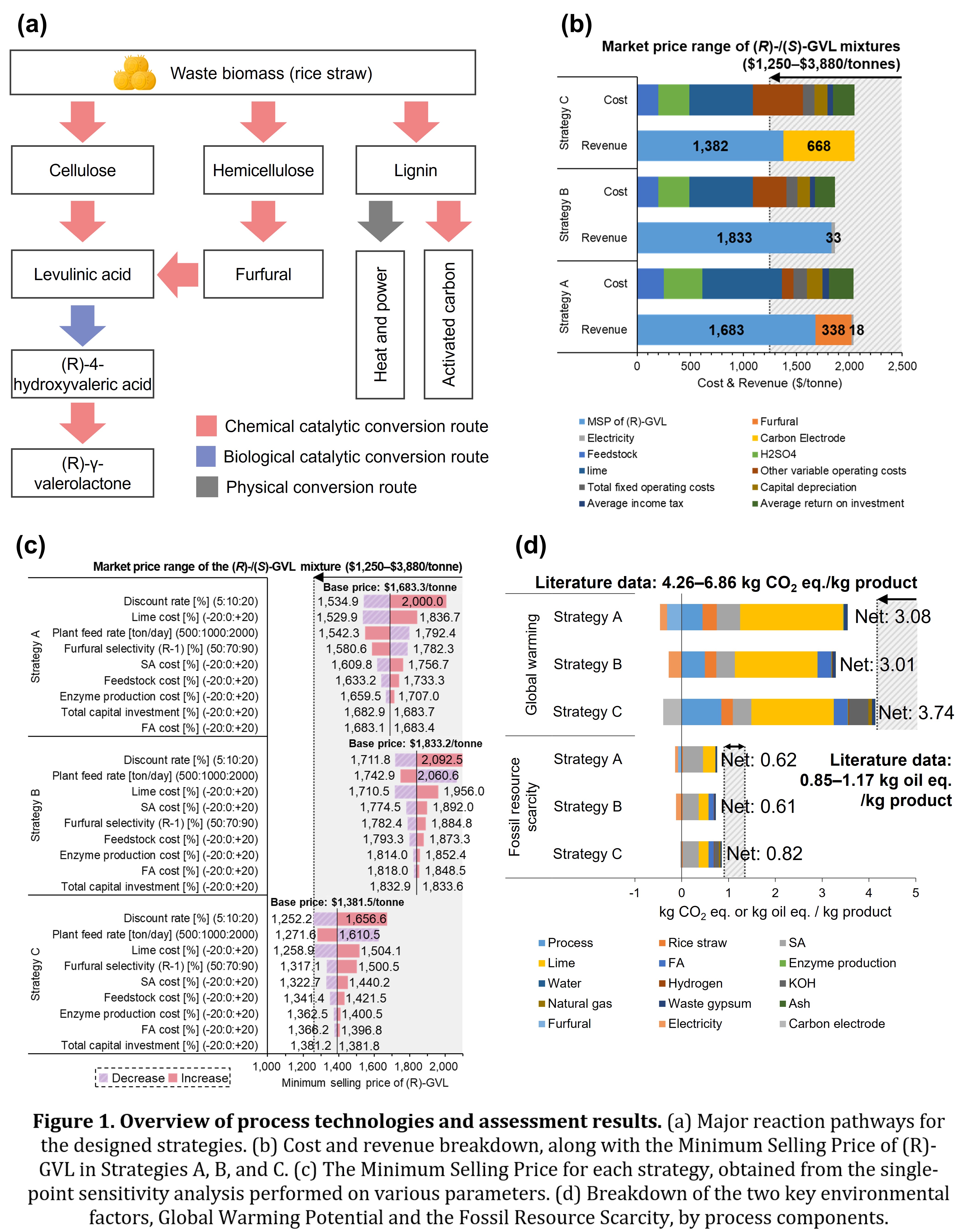
In this study, we propose process strategies with an optimized design rather than developing new catalysts to bridge the gap between two different catalytic reaction conditions. To overcome incompatibility, we developed carbon upgrading strategies. Three different process strategies were designed based on three main components of waste rice straw: cellulose, hemicellulose, and lignin. In Strategy A, only cellulose is utilized for (R)-GVL production, while hemicellulose and lignin are just converted into furfural and electricity, respectively. This strategy serves as the baseline for evaluating the feasibility of the chemo-enzymatic reaction proposed by Cha et al. (in press), incorporating only the essential conversion steps. In Strategy B, however, furfural derived from hemicellulose is converted into levulinic acid, improving the efficiency of (R)-GVL production. Building on this approach, lignin undergoes further refinement to produce high-quality activated carbon, suitable for potential applications in supercapacitors in Strategy C.
After reviewing the literature, we adopted several conversion technologies to implement our strategies. Cha et al. (in press) introduced a one-pot system for converting rice straw into levulinic acid (LA), formic acid (FA), and furfural using dilute sulfuric acid under optimized conditions (170°C, 7.7 atm, 40 minutes). The resulting LA is transformed into (R)-GVL through a chemo-enzymatic process. First, LA is enantioselectively reduced to (R)-4-hydroxyvaleric acid (HV) by the enzyme eHBDH with coenzyme NADH, where the consumed NADH is regenerated by FA. Next, the intermediate (R)-HV is converted to (R)-GVL in an acid-catalyzed reaction at 121°C and 2 atm. Furfural from hemicellulose can be upgraded to LA via Pt3Sn/SiO2 and Amberlyst 70, contributing further to (R)-GVL production (Alonso et al., 2013). Lastly, lignin is either used to generate heat and power or activated into high-surface-area carbon (3,207 m²/g) via KOH and N2 (Yu et al., 2023), making it suitable for adsorbents and high-value supercapacitors.
Based on previous conversion technologies, we developed process models for three strategies to process 1,000 dry tonnes of rice straw per day. For each strategy, we optimized the process design by considering that the amounts of input materials and utilities in the process are proportional to the solvent quantity. Heat integration was performed using a heat exchange network to further reduce utility consumption. The developed process model was then used to calculate material and energy balances, and these results were utilized for techno-economic analysis (TEA) and life-cycle assessment (LCA) to evaluate the feasibility of the process.
To perform the TEA, we used the Aspen Process Economic Analyzer and literature data to calculate capital and operating costs. Based on these cost estimates, we assessed the economic feasibility of each strategy by determining the Minimum Selling Price (MSP) using the discounted cash flow method. The MSPs of Strategy A, B, and C were calculated to be $1,683/tonne, $1,833/tonne, and $1,383/tonne, respectively. Considering the market price range of the (R)-/(S)-GVL mixture is $1,250–$3,880/tonne, all proposed strategies are market-competitive. Compared to Strategy A, Strategy B increased (R)-GVL production by 25% and generated more electricity revenue from humin. However, the higher total costs of the furfural conversion step led to a 9% increase in the MSP. Meanwhile, Strategy C had higher costs due to the additional lignin activation processes and did not sell surplus electricity. Nevertheless, Strategy C significantly lowered its MSP (18% lower than Strategy A) by selling supercapacitor-grade activated carbon at $9,500/tonne.
We performed sensitivity analyses to identify major cost drivers and consider variations in economic conditions and technological advancements for Strategies A, B, and C. In all three strategies, the discount rate, lime cost, and plant feed rate had a significant impact on MSP, while furfural selectivity, sulfuric acid cost, and feedstock had moderate effects. Among these factors, lime is used as a neutralization reagent for an enzymatic reaction, and sulfuric acid is used as a chemical catalyst. This result reveals that improvements in the strategic process design, and the chemo-enzymatic reaction still have an impact on the overall economic feasibility of the process. In Strategy A, increasing furfural selectivity reduced MSP due to extra furfural sales, whereas in Strategy B and C, it raised MSP by boosting catalyst regeneration costs. Further analysis showed that changes in byproduct prices for furfural and activated carbon could shift the lowest MSP strategy between A, B, and C. Strategy C generally offered the lowest MSP among the three strategies when activated carbon was sold at a high price and furfural prices remained stable at around $1,000/tonne, as reported in the literature. However, if furfural prices rose or activated carbon prices fell, Strategy A became more competitive. Overall, the key economic drivers highlight the need to balance process design with both raw material costs and evolving market conditions.
The LCA evaluated the environmental feasibility of the three proposed strategies. The Global Warming Potential (GWP) for Strategy A, B, and C was 3.08, 3.01, and 3.74 kg CO2 eq./kg product, respectively. The Fossil Resource Scarcity (FRS) was 0.62, 0.61, and 0.82 kg oil eq./kg product for Strategy A, B, and C, respectively. Both GWP and FRS, which are key environmental indicators, were lower than previously reported values in the literature (4.26–6.86 kg CO2 eq./kg product for the GWP and 0.85–1.17 kg oil eq./kg product for the FRS). These results were achieved using waste biomass and co-producing electricity, furfural, and activated carbon. According to the breakdown results, lime and sulfuric acid had a significant impact on both GWP and FRS across all strategies. Given lime and sulfuric acid are essential reagents for the chemo-enzymatic reaction, this reaction still has a significant environmental impact.
In conclusion, we developed a process strategy to valorize rice straw using a chemo-enzymatic reaction. Developing new chemical and enzymatic catalysts that can operate under similar reaction conditions continues to pose a significant challenge. Therefore, from a process perspective, we introduced additional feedstock upgrading strategies and optimized process designs for each approach to mitigate the limitations of current chemo-enzymatic reactions. TEA and LCA results demonstrated that the designed processes surpass conventional methods in both economic and environmental aspects. This highlights that a process-oriented approach can enhance the commercial viability of chemo-enzymatic reactions. The insights from this study can be broadly applied to precision chemistry and biomass conversion using chemo-enzymatic processes.
References
Rudroff, Florian, et al. "Opportunities and challenges for combining chemo-and biocatalysis." Nature Catalysis 1.1 (2018): 12-22.
Gröger, Harald, Fabrice Gallou, and Bruce H. Lipshutz. "Where chemocatalysis meets biocatalysis: in water." Chemical Reviews 123.9 (2022): 5262-5296.
Cha, Jaehyun, et al. “Non-sugar biorefineries for agrowaste-derived enantioselective (R)-γ-valerolactone production: technoeconomic analysis and life cycle assessment.” Nature Chemical Engineering (in press).
Alonso, David Martin, et al. "Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts." Catalysis Science & Technology 3.4 (2013): 927-931.
Yu, Lu, et al. "Tailored mesoporous structures of lignin-derived nano-carbons for multiple applications." Carbon 213 (2023): 118285.