2025 AIChE Annual Meeting
(8e) Unravelling the Role of Reaction Environment at the Metal-Reactant Interface for the Targeted Reduction of Aldehydes to Alcohols
Authors
Alyssa Hensley, Stevens Institute of Technology
Zhenwei Wu, University of Toronto, Canada
Ya-Huei (Cathy) Chin, University of Toronto
Jean-Sabin McEwen, Washington State University
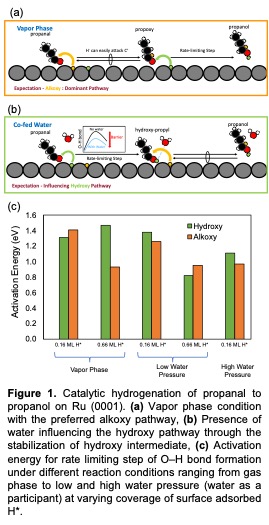
Oxygenated compound such as aldehydes or ketones generated during fast pyrolysis of biomass, can be selectively reduced to produce value-added products like alcohols. Catalytic hydrogenation of these carbonyl compounds on transition metals involves two sequential hydrogen additions onto the C=O bond via two common pathways: (1) Hydroxy, where the O‒H bond forms first or (2) Alkoxy, where the C‒H bond forms first. Here, we employ density functional theory to probe the mechanistic effects of a polar protic solvent (i.e. water) on the energetically dominant reaction pathway for propanal hydrogenation to propanol on Ru (0001). Critical factors influencing the reaction pathways such as a high concentration of reacting species, hydrogen pressure, presence of solvent and undesired side products (CO molecules) is also examined. Our results suggest that, in the vapor phase, hydrogenation occurs via the Alkoxy pathway; the initial C‒H bond formation step is quasi-equilibrated and the secondary O‒H bond formation step is slow and rate-limiting. Increasing the hydrogen concentration in the vapor phase primarily influences the Alkoxy pathway by decreasing the activation energy for O‒H bond formation by 0.5 eV. In the presence of a small amount of water and at high hydrogen concentrations, the preferred hydrogenation pathway shifts to the Hydroxy pathway. We find that the addition of water selectively decreases the activation energy for the Hydroxy pathway’s rate limiting step by 0.6 eV as compared to the vapor phase. A similar trend is observed with increased water concentration. Our results indicate that polar, protic solvent can promote the hydrogenation of carbonyl containing compounds by stabilizing the transition state and intermediates via hydrogen-bonding. Furthermore, these model results are correlated with experimental, hydrogenation kinetic-isotopic analyses, developing accurate atomistic insights into the reaction mechanism and improving aldehyde hydrogenation catalysts.