2025 AIChE Annual Meeting
(404f) Unravelling Insights in Polyol Hydrodeoxygenation Using Reaction Energetics Computed Under Non-Ideal Conditions Using Digital Chemistry
Authors
Tej Choksi - Presenter, Nanyang Technological University
Karam Hashem, Nanyang Technological University
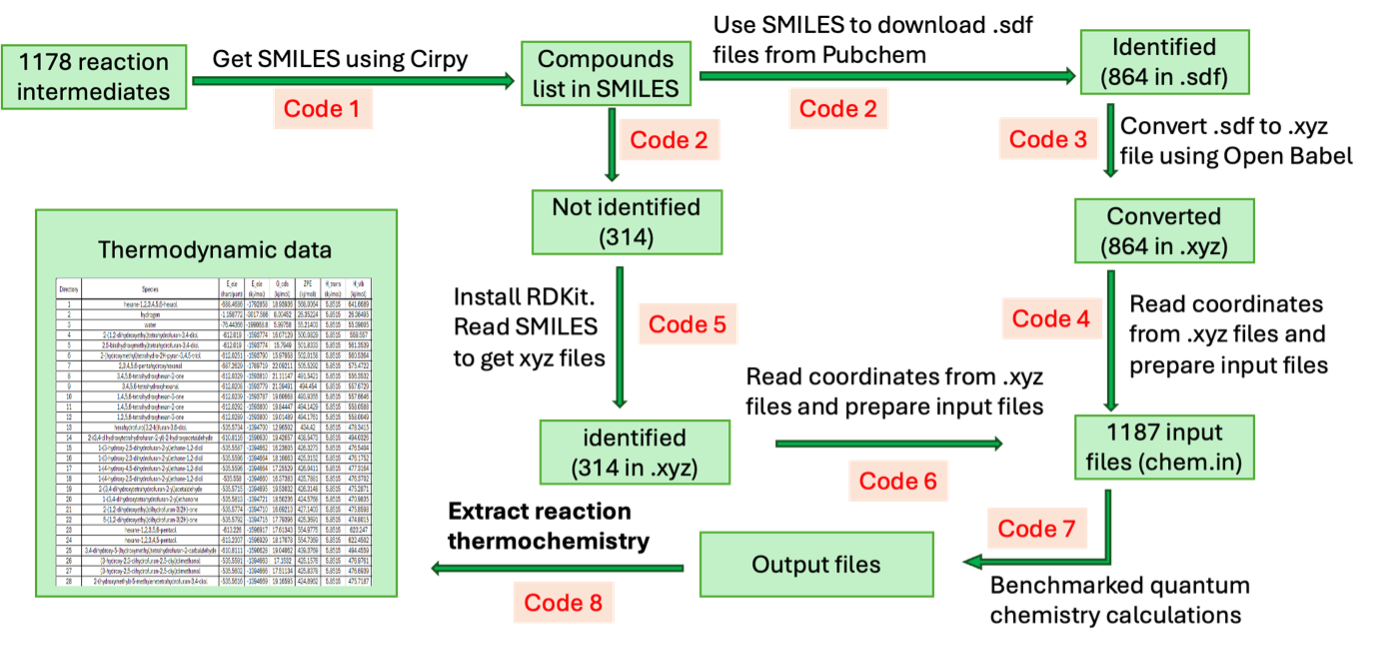
Despite advances in the design of hydrodeoxygenation (HDO) catalysts, the more fundamental questions about reaction energetics, and the reaction mechanism remain unresolved. A rigorous understanding of the reaction energetics during HDO is essential to: (a) determine thermodynamic limitations to HDO products, (b) guide reaction pathway analysis, and (c) determine the heat duty of HDO reactors. Computing enthalpy, entropy, and free energy changes of HDO reactions is challenging because of non-idealities in the aqueous, oil, and gas phase, and the large reaction networks. Most studies, however, ignore these non-idealities and evaluate reaction energetics of HDO using inaccurate ideal gas or group contribution methods. Based on experimentally detected products of sorbitol HDO, Moreno et al. [1] enumerated a mechanism involving 4804 reactions and 1178 distinct chemical intermediates. We establish an automated digital chemistry platform that computes the free energy changes under non-ideal conditions of all 4804 reactions at user-specified conditions. Benchmarked quantum chemistry methods together with implicit and explicit solvation interactions are used to determine the changes in total energies. Statistical thermodynamics yield free energy changes in solvated environments, with gas phase species being simulated using the Peng-Robinson equation of state. Reaction energetics of sorbitol HDO with this glass box platform are compared to outputs from black box phase-equilibrium models in ASPEN Plus, to assess uncertainty in the predictions. The heat released during sorbitol HDO is computed as a function of reaction conditions and catalyst compositions (noble and non-noble metals), thus quantifying the extent of heat integration that is possible. The computed free energies are used to chart out plausible mechanisms during early- and end-stages of sorbitol HDO where competition between dehydration/hydrogenation, dehydrogenation, and retro-aldol prevails. This versatile and streamlined platform will be of interest in both academic and industrial R&D.
[1] Moreno, Li, Lee, Huber, Klein. RSC Adv., 3, 23769, (2013)