2025 AIChE Annual Meeting
(8a) Unraveling the Oxidation of 1,1-Dimethoxyethane: A Combined Experimental and Kinetic Modeling Study
Authors
Samuel Hartness, University of Georgia
Nicholas S. Dewey, University of Georgia
Solmaz Nadiri, Physikalisch-Technische Bundesanstalt
Ravi X. Fernandes, Physikalisch-Technische Bundesanstalt
Brandon Rotavera, University of Georgia
Maarten K. Sabbe, Universiteit Gent
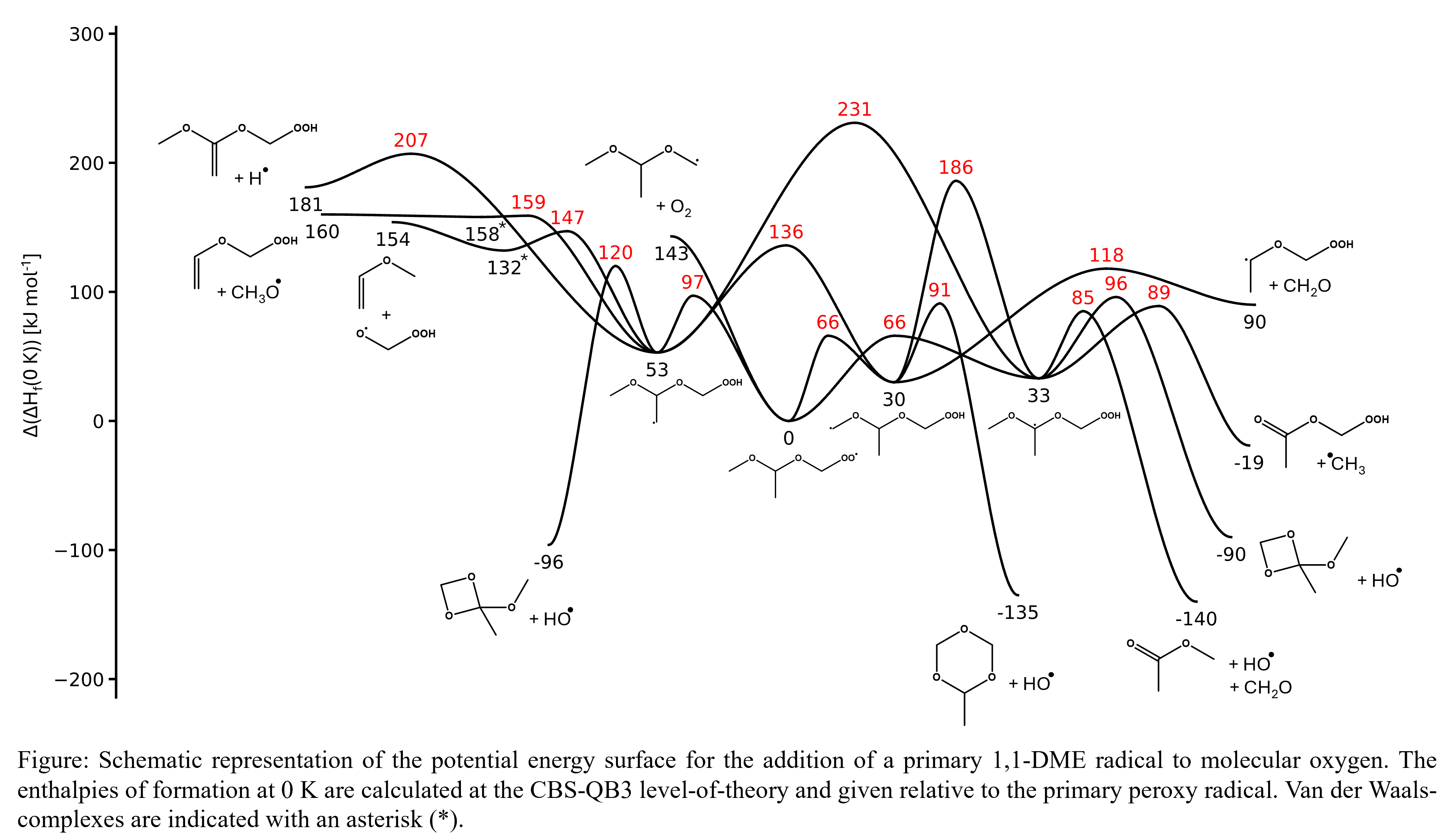
The detrimental effects of global warming on our society are urging the replacement of fossil-based fuels with more sustainable ones. However, due to the limited energy density of batteries, electrification remains impractical for heavy-duty transport, such as ships, airplanes and trucks. In this light, there has been a growing interest in the development and characterization of alternative fuels that can be produced from renewable resources. Oxymethylene ethers (OMEs), species consisting of a backbone of alternating carbon and oxygen atoms, are an example of a high-potential class of e-fuels. Despite their beneficial combustion properties, including a high cetane number and reduced pollutant emissions, they still have a significantly lower energy density than fossil-based fuels. Therefore, in this work, the oxidation chemistry of 1,1-dimethoxyethane (1,1-DME) was studied. Compared to OMEs, 1,1-DME contains a carbon-carbon bond, thereby increasing the energy density. To assess the impact of this carbon-carbon bond on the oxidation chemistry, a detailed kinetic model has been developed. The in-house developed kinetic model generation framework, Genesys, was used for the construction of the kinetic model. The thermodynamic properties and reaction rate coefficients were determined from quantum chemical calculations performed at the CBS-QB3 level-of-theory. These calculations revealed the existence of an important unimolecular decomposition pathway yielding methanol and methyl vinyl ether. Moreover, potential energy surfaces have been constructed to visualize the important decomposition pathways for low-temperature oxidation. These potential energy surfaces, as shown in the figure, highlight the importance of methyl acetate as intermediate. Oxidation experiments have been performed in a jet-stirred reactor over a broad temperature range of 425-900 K for the validation of the kinetic model. The oxidation of 1,1-DME starts above 600 K and reaches full conversion at 900 K. The primary oxidation products included: methanol, acetaldehyde and methyl acetate, which is consistent with the quantum chemical calculations.